Dual thick and thin filament linked regulation of stretch- and L-NAME-induced tone in young and senescent murine basilar artery
- PMID: 37057180
- PMCID: PMC10088910
- DOI: 10.3389/fphys.2023.1099278
Dual thick and thin filament linked regulation of stretch- and L-NAME-induced tone in young and senescent murine basilar artery
Abstract
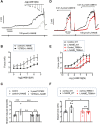
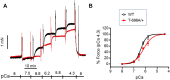
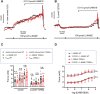
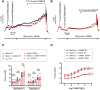
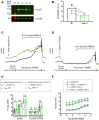
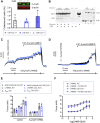
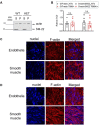
Stretch-induced vascular tone is an important element of autoregulatory adaptation of cerebral vasculature to maintain cerebral flow constant despite changes in perfusion pressure. Little is known as to the regulation of tone in senescent basilar arteries. We tested the hypothesis, that thin filament mechanisms in addition to smooth muscle myosin-II regulatory-light-chain-(MLC20)-phosphorylation and non-muscle-myosin-II, contribute to regulation of stretch-induced tone. In young BAs (y-BAs) mechanical stretch does not lead to spontaneous tone generation. Stretch-induced tone in y-BAs appeared only after inhibition of NO-release by L-NAME and was fully prevented by treatment with 3 μmol/L RhoA-kinase (ROK) inhibitor Y27632. L-NAME-induced tone was reduced in y-BAs from heterozygous mice carrying a point mutation of the targeting-subunit of the myosin phosphatase, MYPT1 at threonine696 (MYPT1-T696A/+). In y-BAs, MYPT1-T696A-mutation also blunted the ability of L-NAME to increase MLC20-phosphorylation. In contrast, senescent BAs (s-BAs; >24 months) developed stable spontaneous stretch-induced tone and pharmacological inhibition of NO-release by L-NAME led to an additive effect. In s-BAs the MYPT1-T696A mutation also blunted MLC20-phosphorylation, but did not prevent development of stretch-induced tone. In s-BAs from both lines, Y27632 completely abolished stretch- and L-NAME-induced tone. In s-BAs phosphorylation of non-muscle-myosin-S1943 and PAK1-T423, shown to be down-stream effectors of ROK was also reduced by Y27632 treatment. Stretch- and L-NAME tone were inhibited by inhibition of non-muscle myosin (NM-myosin) by blebbistatin. We also tested whether the substrate of PAK1 the thin-filament associated protein, caldesmon is involved in the regulation of stretch-induced tone in advanced age. BAs obtained from heterozygotes Cald1+/- mice generated stretch-induced tone already at an age of 20-21 months old BAs (o-BA). The magnitude of stretch-induced tone in Cald1+/- o-BAs was similar to that in s-BA. In addition, truncation of caldesmon myosin binding Exon2 (CaD-▵Ex2-/-) did not accelerate stretch-induced tone. Our study indicates that in senescent cerebral vessels, mechanisms distinct from MLC20 phosphorylation contribute to regulation of tone in the absence of a contractile agonist. While in y-and o-BA the canonical pathways, i.e., inhibition of MLCP by ROK and increase in pMLC20, predominate, tone regulation in senescence involves ROK regulated mechanisms, involving non-muscle-myosin and thin filament linked mechanisms involving caldesmon.
Keywords: basilar artery; caldesmon; non-muscle myosin; senescence; stretch-induced tone.
Copyright © 2023 Lubomirov, Schroeter, Hasse, Frohn, Metzler, Bust, Pryymachuk, Hescheler, Grisk, Chalovich, Smyth, Pfitzer and Papadopoulos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Artamonov M. V., Sonkusare S. K., Good M. E., Momotani K., Eto M., Isakson B. E., et al. (2018). RSK2 contributes to myogenic vasoconstriction of resistance arteries by activating smooth muscle myosin and the Na(+)/H(+) exchanger. Sci. Signal 11 (554), eaar3924. 10.1126/scisignal.aar3924 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

