Preparation, Characterization and ex vivo Skin Permeability Evaluation of Type I Collagen-Loaded Liposomes
- PMID: 37057190
- PMCID: PMC10086223
- DOI: 10.2147/IJN.S404494
Preparation, Characterization and ex vivo Skin Permeability Evaluation of Type I Collagen-Loaded Liposomes
Abstract
Purpose: In the present study, we prepared collagen liposomes with the addition of polyol, which is expected to not only increase the solubility of collagen but also improve skin penetration.
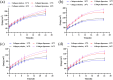
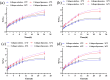
Methods: Collagen liposomes were prepared by the film dispersion method, and their characteristics, integrity and biosafety were evaluated by Fourier transform infrared spectroscopy (FTIR), UV-VIS spectroscopy, polyacrylamide gel electrophoresis (SDS-PAGE), dynamic light scattering (DLS) and transmission electron microscope (TEM). The transdermal absorption of collagen and collagen liposomes were tested by an ex vivo horizontal Valia-Chien diffusion cell system.
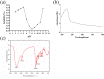
Results: We first demonstrated that collagen extracted from bovine Achilles tendon was type I collagen. The results of DLS measurement and TEM observation showed that the collagen liposomes were spherical in shape with average diameter (75.34±0.93 nm) and maintained high stability at low temperature (4°C) for at least 42 days without toxicity. The encapsulation rate of collagen liposomes was 57.80 ± 0.51%, and SDS-PAGE analysis showed that collagen was intact in liposomes. Finally, permeability studies indicated that the collagen-loaded liposomes more easily penetrated the skin compared to collagen itself.
Conclusion: This study proposed a new method to improve the bioavailability and permeability of bovine type I collagen, which improves the applicability of collagen in biomedicine, cosmeceuticals and pharmaceutical industries.
Keywords: collagen; drug delivery; liposome; physicochemical properties of collagen; skin penetration.
© 2023 Li et al.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Figures







References
-
- Tang C, Zhou K, Zhu YC, et al. Collagen and its derivatives: from structure and properties to their applications in food industry. Food Hydrocoll. 2022;131:107748. doi:10.1016/j.foodhyd.2022.107748 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

