MHC class I and MHC class II reporter mice enable analysis of immune oligodendroglia in mouse models of multiple sclerosis
- PMID: 37057892
- PMCID: PMC10181822
- DOI: 10.7554/eLife.82938
MHC class I and MHC class II reporter mice enable analysis of immune oligodendroglia in mouse models of multiple sclerosis
Abstract
Oligodendrocytes and their progenitors upregulate MHC pathways in response to inflammation, but the frequency of this phenotypic change is unknown and the features of these immune oligodendroglia are poorly defined. We generated MHC class I and II transgenic reporter mice to define their dynamics in response to inflammatory demyelination, providing a means to monitor MHC activation in diverse cell types in living mice and define their roles in aging, injury, and disease.
Keywords: immunology; inflammation; major histocompatibility complex; mouse; multiple sclerosis; neuroscience; oligodendrocytes.
Plain language summary
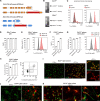
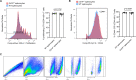
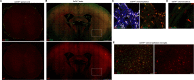
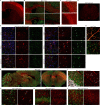
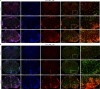
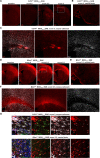
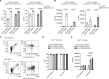
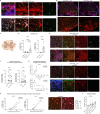
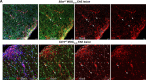
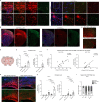
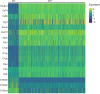
Nerve cells in the brain and spinal cord are surrounded by a layer of insulation called myelin that allows cells to transmit messages to each other more quickly and efficiently. This protective sheath is produced by cells called oligodendrocytes which together with their immature counterparts can also repair damage caused to myelin. In the inflammatory disease multiple sclerosis (MS), this insulation is disrupted and oligodendroglia fail to repair breaks in the myelin sheath, leaving nerves vulnerable to further damage. Recently it was discovered that mature and immature oligodendrocytes (which are collectively known as oligodendroglia) sometimes express proteins normally restricted to the immune system called major histocompatibility complexes (or MHCs for short). Researchers believe that MHC expression may allow oligodendroglia to interact with immune cells, potentially leading to the removal of oligodendroglia by the immune system as well as inflammation that exacerbates damage to nerves and hinders myelin repair. Knowing when oligodendroglia start producing MHCs and where these MHC-expressing cells are located is therefore important for understanding their role in MS. However, it is difficult to identify the location of MHC-expressing oligodendroglia using methods that are currently available. To address this, Harrington, Catenacci et al. created a genetically engineered mouse model in which the MHC-expressing oligodendroglia also generated a red fluorescent protein that could be detected under a microscope. This revealed that only a small number of oligodendroglia in the nervous system had MHCs, but these cells were located in areas of the brain and spinal cord with the highest inflammatory activity. Further microscopy studies in mice that developed MS-like symptoms revealed that MHC production in oligodendroglia increased compared with healthy animals, and that the proportion of oligodendroglia that produced MHC was highest in mice with the most severe symptoms. MHC-expressing oligodendroglia also congregated in the most damaged areas of the brain and spinal cord. These results suggest that MHC expression may contribute to inflammation and impact the function of oligodendroglia that have these molecules. In the future, Harrington et al. hope that their new mouse model will help researchers study the role of MHC expression in different diseases, and in the case of MS, aid the development of new treatments.
© 2023, Harrington, Catenacci et al.
Conflict of interest statement
EH, RC, MS, DH, CM, KM, JG, DB, PC No competing interests declared
Figures









Update of
References
-
- Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin JP, Wu T, Johnson KR, McGavern DB, Schafer DP, Calabresi PA, Reich DS. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597:709–714. doi: 10.1038/s41586-021-03892-7. - DOI - PMC - PubMed
-
- Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, Chak S, Naikawadi RP, Wolters PJ, Abate AR, Butte AJ, Bhattacharya M. Reference-Based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nature Immunology. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. - DOI - PMC - PubMed
-
- Baxi EG, DeBruin J, Tosi DM, Grishkan IV, Smith MD, Kirby LA, Strasburger HJ, Fairchild AN, Calabresi PA, Gocke AR. Transfer of myelin-reactive Th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. The Journal of Neuroscience. 2015;35:8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. - DOI - PMC - PubMed
-
- Benayoun BA, Pollina EA, Singh PP, Mahmoudi S, Harel I, Casey KM, Dulken BW, Kundaje A, Brunet A. Remodeling of epigenome and transcriptome landscapes with aging in mice reveals widespread induction of inflammatory responses. Genome Research. 2019;29:697–709. doi: 10.1101/gr.240093.118. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

