A cell-free antigen processing system informs HIV-1 epitope selection and vaccine design
- PMID: 37058141
- PMCID: PMC10114365
- DOI: 10.1084/jem.20221654
A cell-free antigen processing system informs HIV-1 epitope selection and vaccine design
Abstract
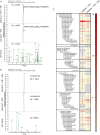
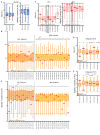
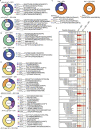
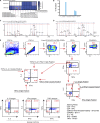
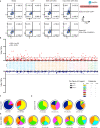
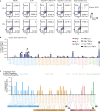
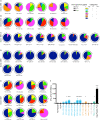
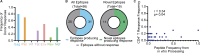
Distinct CD4+ T cell epitopes have been associated with spontaneous control of HIV-1 replication, but analysis of antigen-dependent factors that influence epitope selection is lacking. To examine these factors, we used a cell-free antigen processing system that incorporates soluble HLA-DR (DR1), HLA-DM (DM), cathepsins, and full-length protein antigens for epitope identification by LC-MS/MS. HIV-1 Gag, Pol, Env, Vif, Tat, Rev, and Nef were examined using this system. We identified 35 novel epitopes, including glycopeptides. Epitopes from smaller HIV-1 proteins mapped to regions of low protein stability and higher solvent accessibility. HIV-1 antigens associated with limited CD4+ T cell responses were processed efficiently, while some protective epitopes were inefficiently processed. 55% of epitopes obtained from cell-free processing induced memory CD4+ T cell responses in HIV-1+ donors, including eight of 19 novel epitopes tested. Thus, an in vitro processing system utilizing the components of Class II processing reveals factors influencing epitope selection of HIV-1 and represents an approach to understanding epitope selection from non-HIV-1 antigens.
© 2023 Sengupta et al.
Conflict of interest statement
Disclosures: S.G. Deeks reported personal fees from Tendel during the conduct of the study. No other disclosures were reported.
Figures







Similar articles
-
Characterization of HIV-specific CD4+ T cell responses against peptides selected with broad population and pathogen coverage.PLoS One. 2012;7(7):e39874. doi: 10.1371/journal.pone.0039874. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792193 Free PMC article.
-
Virus-like vaccines against HIV/SIV synergize with a subdominant antigen T cell vaccine.J Transl Med. 2019 May 24;17(1):175. doi: 10.1186/s12967-019-1924-1. J Transl Med. 2019. PMID: 31126293 Free PMC article.
-
In silico design and in vitro expression of novel multiepitope DNA constructs based on HIV-1 proteins and Hsp70 T-cell epitopes.Biotechnol Lett. 2021 Aug;43(8):1513-1550. doi: 10.1007/s10529-021-03143-9. Epub 2021 May 13. Biotechnol Lett. 2021. PMID: 33987776 Free PMC article.
-
Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1077-88. doi: 10.1089/AID.2015.0101. Epub 2015 Aug 13. AIDS Res Hum Retroviruses. 2015. PMID: 26149745 Review.
-
Antigen-presenting cells and the selection of immunodominant epitopes.Crit Rev Immunol. 1997;17(5-6):411-7. Crit Rev Immunol. 1997. PMID: 9419428 Review.
Cited by
-
Exploratory algorithms to devise multi-epitope subunit vaccine by examining HIV-1 envelope glycoprotein: An immunoinformatics and viroinformatics approach.PLoS One. 2025 Feb 27;20(2):e0318523. doi: 10.1371/journal.pone.0318523. eCollection 2025. PLoS One. 2025. Retraction in: PLoS One. 2025 May 6;20(5):e0324076. doi: 10.1371/journal.pone.0324076. PMID: 40014623 Free PMC article. Retracted.
-
T-Cell Epitope-Based Vaccines: A Promising Strategy for Prevention of Infectious Diseases.Vaccines (Basel). 2024 Oct 17;12(10):1181. doi: 10.3390/vaccines12101181. Vaccines (Basel). 2024. PMID: 39460347 Free PMC article. Review.
-
Harnessing Autophagy to Overcome Antigen-Specific T-Cell Dysfunction: Implication for People Living with HIV-1.Int J Mol Sci. 2023 Jul 3;24(13):11018. doi: 10.3390/ijms241311018. Int J Mol Sci. 2023. PMID: 37446195 Free PMC article. Review.
-
Proper development of long-lived memory CD4 T cells requires HLA-DO function.Front Immunol. 2023 Oct 16;14:1277609. doi: 10.3389/fimmu.2023.1277609. eCollection 2023. Front Immunol. 2023. PMID: 37908352 Free PMC article.
References
-
- Abelin, J.G., Keskin D.B., Sarkizova S., Hartigan C.R., Zhang W., Sidney J., Stevens J., Lane W., Zhang G.L., Eisenhaure T.M., et al. . 2017. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity. 46:315–326. 10.1016/j.immuni.2017.02.007 - DOI - PMC - PubMed
-
- Altfeld, M., Rosenberg E.S., Shankarappa R., Mukherjee J.S., Hecht F.M., Eldridge R.L., Addo M.M., Poon S.H., Phillips M.N., Robbins G.K., et al. . 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169–180. 10.1084/jem.193.2.169 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

