Estimated Costs and Outcomes Associated With Use and Nonuse of Medications for Opioid Use Disorder During Incarceration and at Release in Massachusetts
- PMID: 37058306
- PMCID: PMC10105308
- DOI: 10.1001/jamanetworkopen.2023.7036
Estimated Costs and Outcomes Associated With Use and Nonuse of Medications for Opioid Use Disorder During Incarceration and at Release in Massachusetts
Abstract
Importance: Most prisons and jails in the US discontinue medications for opioid use disorder (MOUD) upon incarceration and do not initiate MOUD prior to release.
Objective: To model the association of MOUD access during incarceration and at release with population-level overdose mortality and OUD-related treatment costs in Massachusetts.
Design, setting, and participants: This economic evaluation used simulation modeling and cost-effectiveness with costs and quality-adjusted life-years (QALYs) discounted at 3% to compare MOUD treatment strategies in a corrections cohort and an open cohort representing individuals with OUD in Massachusetts. Data were analyzed between July 1, 2021, and September 30, 2022.
Exposures: Three strategies were compared: (1) no MOUD provided during incarceration or at release, (2) extended-release (XR) naltrexone offered only at release from incarceration, and (3) all 3 MOUDs (naltrexone, buprenorphine, and methadone) offered at intake.
Main outcomes and measures: Treatment starts and retention, fatal overdoses, life-years and QALYs, costs, and incremental cost-effectiveness ratios (ICERs).
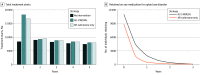
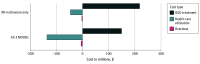
Results: Among 30 000 simulated incarcerated individuals with OUD, offering no MOUD was associated with 40 927 (95% uncertainty interval [UI], 39 001-42 082) MOUD treatment starts over a 5-year period and 1259 (95% UI, 1130-1323) overdose deaths after 5 years. Over 5 years, offering XR-naltrexone at release led to 10 466 (95% UI, 8515-12 201) additional treatment starts, 40 (95% UI, 16-50) fewer overdose deaths, and 0.08 (95% UI, 0.05-0.11) QALYs gained per person, at an incremental cost of $2723 (95% UI, $141-$5244) per person. In comparison, offering all 3 MOUDs at intake led to 11 923 (95% UI, 10 861-12 911) additional treatment starts, compared with offering no MOUD, 83 (95% UI, 72-91) fewer overdose deaths, and 0.12 (95% UI, 0.10-0.17) QALYs per person gained, at an incremental cost of $852 (95% UI, $14-$1703) per person. Thus, XR-naltrexone only was a dominated strategy (both less effective and more costly) and the ICER of all 3 MOUDs compared with no MOUD was $7252 (95% UI, $140-$10 018) per QALY. Among everyone with OUD in Massachusetts, XR-naltrexone only averted 95 overdose deaths over 5 years (95% UI, 85-169)-a 0.9% decrease in state-level overdose mortality-while the all-MOUD strategy averted 192 overdose deaths (95% UI, 156-200)-a 1.8% decrease.
Conclusions and relevance: The findings of this simulation-modeling economic study suggest that offering any MOUD to incarcerated individuals with OUD would prevent overdose deaths and that offering all 3 MOUDs would prevent more deaths and save money compared with an XR-naltrexone-only strategy.
Conflict of interest statement
Figures
Comment in
-
Is Providing Medications for Opioid Use Disorder to Incarcerated Individuals a Cost-effective Strategy?JAMA Netw Open. 2023 Apr 3;6(4):e237001. doi: 10.1001/jamanetworkopen.2023.7001. JAMA Netw Open. 2023. PMID: 37058311 No abstract available.
Similar articles
-
Expanding access to Medication for Opioid Use Disorder (MOUD) in jails: A comprehensive program evaluation.J Subst Use Addict Treat. 2024 Jun;161:209248. doi: 10.1016/j.josat.2023.209248. Epub 2023 Dec 9. J Subst Use Addict Treat. 2024. PMID: 38081540
-
Massachusetts Justice Community Opioid Innovation Network (MassJCOIN).J Subst Abuse Treat. 2021 Sep;128:108275. doi: 10.1016/j.jsat.2021.108275. Epub 2021 Jan 8. J Subst Abuse Treat. 2021. PMID: 33483222 Free PMC article.
-
Cost-effectiveness of extended-release injectable naltrexone among incarcerated persons with opioid use disorder before release from prison versus after release.J Subst Abuse Treat. 2022 Oct;141:108835. doi: 10.1016/j.jsat.2022.108835. Epub 2022 Jul 2. J Subst Abuse Treat. 2022. PMID: 35933942 Free PMC article. Clinical Trial.
-
Opioid-related treatment, interventions, and outcomes among incarcerated persons: A systematic review.PLoS Med. 2019 Dec 31;16(12):e1003002. doi: 10.1371/journal.pmed.1003002. eCollection 2019 Dec. PLoS Med. 2019. PMID: 31891578 Free PMC article.
-
Access to Medications for Opioid Use Disorder and Associated Factors Among Adolescents and Young Adults: A Systematic Review.JAMA Pediatr. 2022 Mar 1;176(3):304-311. doi: 10.1001/jamapediatrics.2021.4606. JAMA Pediatr. 2022. PMID: 34870707 Free PMC article.
Cited by
-
Responding to the US opioid crisis: leveraging analytics to support decision making.Health Care Manag Sci. 2023 Dec;26(4):599-603. doi: 10.1007/s10729-023-09657-0. Epub 2023 Oct 7. Health Care Manag Sci. 2023. PMID: 37804456 Free PMC article.
-
The importance of contextually specific support relationships in implementing programs to link people to medication for opioid use disorder (MOUD) treatment during reentry from county jails.Health Justice. 2025 Mar 31;13(1):20. doi: 10.1186/s40352-025-00330-y. Health Justice. 2025. PMID: 40163160 Free PMC article.
-
Health and Economic Outcomes of Offering Buprenorphine in Homeless Shelters in Massachusetts.JAMA Netw Open. 2024 Oct 1;7(10):e2437233. doi: 10.1001/jamanetworkopen.2024.37233. JAMA Netw Open. 2024. PMID: 39412807 Free PMC article.
-
Simulated impact of medicaid expansion on the economic burden of opioid use disorder in North Carolina.Int J Drug Policy. 2024 Jun;128:104449. doi: 10.1016/j.drugpo.2024.104449. Epub 2024 May 10. Int J Drug Policy. 2024. PMID: 38733650 Free PMC article.
-
Agent-Based Model of Combined Community- and Jail-Based Take-Home Naloxone Distribution.JAMA Netw Open. 2024 Dec 2;7(12):e2448732. doi: 10.1001/jamanetworkopen.2024.48732. JAMA Netw Open. 2024. PMID: 39656460 Free PMC article.
References
-
- Centers for Disease Control and Prevention . Leading Causes of Death and Injury. Reviewed January 19, 2023. Accessed June 2, 2020. https://www.cdc.gov/injury/wisqars/LeadingCauses.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

