Regulation of Monocyte Activation by PPARα Through Interaction With the cGAS-STING Pathway
- PMID: 37058417
- PMCID: PMC10281240
- DOI: 10.2337/db22-0654
Regulation of Monocyte Activation by PPARα Through Interaction With the cGAS-STING Pathway
Abstract
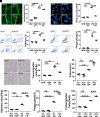
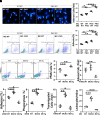
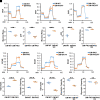
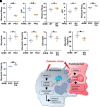
Monocyte activation plays an important role in diabetic complications such as diabetic retinopathy (DR). However, the regulation of monocyte activation in diabetes remains elusive. Fenofibrate, an agonist of peroxisome proliferator-activated receptor-α (PPARα), has shown robust therapeutic effects on DR in patients with type 2 diabetes. Here we found that PPARα levels were significantly downregulated in monocytes from patients with diabetes and animal models, correlating with monocyte activation. Fenofibrate attenuated monocyte activation in diabetes, while PPARα knockout alone induced monocyte activation. Furthermore, monocyte-specific PPARα overexpression ameliorated, while monocyte-specific PPARα knockout aggravated monocyte activation in diabetes. PPARα knockout impaired mitochondrial function while also increasing glycolysis in monocytes. PPARα knockout increased cytosolic mitochondrial DNA release and activation of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway in monocytes under diabetic conditions. STING knockout or STING inhibitor attenuated monocyte activation induced by diabetes or by PPARα knockout. These observations suggest that PPARα negatively regulates monocyte activation through metabolic reprogramming and interaction with the cGAS-STING pathway.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures



Similar articles
-
Modulation of cGAS-STING signaling by PPARα in a mouse model of ischemia-induced retinopathy.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2208934119. doi: 10.1073/pnas.2208934119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409895 Free PMC article.
-
Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina.Am J Pathol. 2014 Oct;184(10):2709-20. doi: 10.1016/j.ajpath.2014.06.021. Epub 2014 Aug 7. Am J Pathol. 2014. PMID: 25108226 Free PMC article.
-
Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models.Diabetes. 2013 Jan;62(1):261-72. doi: 10.2337/db11-0413. Epub 2012 Oct 5. Diabetes. 2013. PMID: 23043158 Free PMC article.
-
PPARα: An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases.Front Endocrinol (Lausanne). 2022 Dec 16;13:1074911. doi: 10.3389/fendo.2022.1074911. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36589809 Free PMC article. Review.
-
The cGAS-STING Pathway: Novel Perspectives in Liver Diseases.Front Immunol. 2021 Apr 29;12:682736. doi: 10.3389/fimmu.2021.682736. eCollection 2021. Front Immunol. 2021. PMID: 33995425 Free PMC article. Review.
Cited by
-
The cGAS-STING pathway: a therapeutic target in diabetes and its complications.Burns Trauma. 2024 Feb 2;12:tkad050. doi: 10.1093/burnst/tkad050. eCollection 2024. Burns Trauma. 2024. PMID: 38312740 Free PMC article. Review.
-
Novel Therapeutic Approaches for Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration.Vision (Basel). 2025 Apr 17;9(2):35. doi: 10.3390/vision9020035. Vision (Basel). 2025. PMID: 40265403 Free PMC article. Review.
-
Role of cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes pathway in diabetes and its complications.World J Diabetes. 2024 Oct 15;15(10):2041-2057. doi: 10.4239/wjd.v15.i10.2041. World J Diabetes. 2024. PMID: 39493568 Free PMC article. Review.
-
Long-Term Minocycline Treatment Exhibits Enhanced Therapeutic Effects on Ischemic Stroke by Suppressing Inflammatory Phenotype of Microglia Through the EMB/MCT4/STING Pathway.CNS Neurosci Ther. 2025 Mar;31(3):e70328. doi: 10.1111/cns.70328. CNS Neurosci Ther. 2025. PMID: 40135489 Free PMC article.
References
-
- Mesquida M, Drawnel F, Fauser S. The role of inflammation in diabetic eye disease. Semin Immunopathol 2019;41:427–445 - PubMed
-
- Binet F, Cagnone G, Crespo-Garcia S, et al. . Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020;369:eaay5356. - PubMed
-
- Rezzola S, Corsini M, Chiodelli P, et al. . Inflammation and N-formyl peptide receptors mediate the angiogenic activity of human vitreous humour in proliferative diabetic retinopathy. Diabetologia 2017;60:719–728 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

