Preliminary construct validity of patient-reported outcomes to assess chronic pain in adults with sickle cell disease
- PMID: 37058480
- PMCID: PMC10365933
- DOI: 10.1182/bloodadvances.2023009707
Preliminary construct validity of patient-reported outcomes to assess chronic pain in adults with sickle cell disease
Abstract
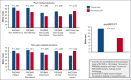
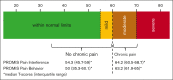
Chronic pain affects 30% to 40% of individuals with sickle cell disease (SCD) and impairs patient functioning. Clinically meaningful, practical, and valid assessment tools for investigation, evaluation, and management of chronic pain are limited, representing a barrier for advancing SCD care. We sought to determine whether patient-reported outcomes (PROs) show preliminary construct validity in identifying individuals with SCD who were a priori defined as suggestive of having chronic pain based on previously published criteria. All individuals completed the Patient-Reported Outcomes Measurement Information System (PROMIS) domains: pain interference, pain behavior, pain quality (nociceptive, neuropathic), fatigue, sleep disturbance, depression, and anxiety; the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) domains: pain impact and emotional impact; and the painDETECT questionnaire. Thirty-three adults living with SCD were enrolled, and 42.4% had chronic pain. Pain-related PROs scores distinctly differentiated individuals with chronic pain from those without. Individuals with chronic pain had significantly worse pain-related PROs scores: PROMIS pain interference (64.2 vs 54.3), PROMIS pain behavior (63.2 vs 50), and ASCQ-Me pain impact (42.9 vs 53.2). According to published PROMIS clinical cut scores for the pain-related domains, individuals with chronic pain were categorized as having moderate impairment, whereas those without chronic pain had mild or no impairment. Individuals with chronic pain had PRO pain features consistent with neuropathic pain and worse scores in fatigue, depression, sleep disturbance, and emotional impact. Pain-related PROs show preliminary construct validity in differentiating individuals with and without chronic SCD pain and could be used as valuable tools for research and clinical monitoring of chronic pain.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.S.H. is a consultant for GBT, Forma Therapeutics, and CVS Health. J.J.F. received research funding from Forma, Shire, and Vifor and is on the data and safety monitoring board for Bayer. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease: comparison of PROMIS® to ASCQ-Me℠.Health Qual Life Outcomes. 2017 Jun 2;15(1):117. doi: 10.1186/s12955-017-0661-5. Health Qual Life Outcomes. 2017. PMID: 28577358 Free PMC article.
-
Patient reports of health outcome for adults living with sickle cell disease: development and testing of the ASCQ-Me item banks.Health Qual Life Outcomes. 2014 Aug 22;12:125. doi: 10.1186/s12955-014-0125-0. Health Qual Life Outcomes. 2014. PMID: 25146160 Free PMC article. Clinical Trial.
-
Patient-reported outcomes in sickle cell disease and association with clinical and psychosocial factors: Report from the sickle cell disease implementation consortium.Am J Hematol. 2020 Sep;95(9):1066-1074. doi: 10.1002/ajh.25880. Epub 2020 Jun 29. Am J Hematol. 2020. PMID: 32449965 Free PMC article. Clinical Trial.
-
What is the future of patient-reported outcomes in sickle-cell disease?Expert Rev Hematol. 2020 Nov;13(11):1165-1173. doi: 10.1080/17474086.2020.1830370. Epub 2020 Oct 15. Expert Rev Hematol. 2020. PMID: 33034214 Free PMC article. Review.
-
Neuropathic Pain and Sickle Cell Disease: a Review of Pharmacologic Management.Curr Pain Headache Rep. 2020 Jul 24;24(9):52. doi: 10.1007/s11916-020-00885-5. Curr Pain Headache Rep. 2020. PMID: 32705357 Review.
Cited by
-
The use of abstract animations and a graphical body image for assessing pain outcomes among adults with sickle cell disease.J Pain. 2025 Jan;26:104720. doi: 10.1016/j.jpain.2024.104720. Epub 2024 Oct 22. J Pain. 2025. PMID: 39447944 Clinical Trial.
-
High-impact chronic pain in sickle cell disease: insights from the Pain in Sickle Cell Epidemiology Study (PiSCES).Pain. 2024 Oct 1;165(10):2364-2369. doi: 10.1097/j.pain.0000000000003262. Epub 2024 May 23. Pain. 2024. PMID: 38787626 Free PMC article.
-
A Patient Centric Model for Vaso-Occlusive Crises in Sickle Cell Disease-Outcomes of a Consensus Exercise Conducted Across Patients and Experts.Clin Transl Sci. 2025 Apr;18(4):e70197. doi: 10.1111/cts.70197. Clin Transl Sci. 2025. PMID: 40135919 Free PMC article.
-
A neuropathic pain scale is effective in identifying neuropathic pain.Am J Transl Res. 2025 Mar 15;17(3):2094-2102. doi: 10.62347/BXRB6015. eCollection 2025. Am J Transl Res. 2025. PMID: 40226007 Free PMC article.
-
What interval of daily pain assessment is required to reliably diagnose chronic pain in SCD? The Pain in Sickle Cell Epidemiology Study.J Sick Cell Dis. 2024 Oct 23;1(1):yoae011. doi: 10.1093/jscdis/yoae011. eCollection 2024. J Sick Cell Dis. 2024. PMID: 40304011 Free PMC article.
References
-
- Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer. 2012;59(2):377–385. - PubMed
-
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease -- life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. - PubMed
-
- Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin J Pain. 2016;32(6):527–533. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

