Probing microstructural changes in muscles of leptin-deficient zebrafish by non-invasive ex-vivo magnetic resonance microimaging
- PMID: 37058498
- PMCID: PMC10104282
- DOI: 10.1371/journal.pone.0284215
Probing microstructural changes in muscles of leptin-deficient zebrafish by non-invasive ex-vivo magnetic resonance microimaging
Abstract
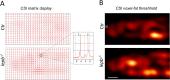
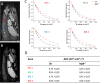
Leptin is a hormone that plays a key role in controlling food intake and energy homeostasis. Skeletal muscle is an important target for leptin and recent studies have shown that leptin deficiency may lead to muscular atrophy. However, leptin deficiency-induced structural changes in muscles are poorly understood. The zebrafish has emerged as an excellent model organism for studies of vertebrate diseases and hormone response mechanisms. In this study, we explored ex-vivo magnetic resonance microimaging (μMRI) methods to non-invasively assess muscle wasting in leptin-deficient (lepb-/-) zebrafish model. The fat mapping performed by using chemical shift selective imaging shows significant fat infiltration in muscles of lepb-/- zebrafish compared to control zebrafish. T2 relaxation measurements show considerably longer T2 values in the muscle of lepb-/- zebrafish. Multiexponential T2 analysis detected a significantly higher value and magnitude of long T2 component in the muscles of lepb-/- as compared to control zebrafish. For further zooming into the microstructural changes, we applied diffusion-weighted MRI. The results show a significant decrease in the apparent diffusion coefficient indicating increased constraints of molecular movements within the muscle regions of lepb-/- zebrafish. The use of the phasor transformation for the separation of diffusion-weighted decay signals showed a bi-component diffusion system which allows us to estimate each fraction on a voxel-wise basis. A substantial difference was found between the ratio of two components in lepb-/- and control zebrafish muscles, indicating alterations in diffusion behavior associated with the tissue microstructural changes in muscles of lepb-/- zebrafish as compared to control zebrafish. Taken together, our results demonstrate that the muscles of lepb-/- zebrafish undergo significant fat infiltration and microstructural changes leading to muscle wasting. This study also demonstrates that μMRI provides excellent means to non-invasively study the microstructural changes in the muscles of the zebrafish model.
Copyright: © 2023 Eeza et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Leptin deficiency affects glucose homeostasis and results in adiposity in zebrafish.J Endocrinol. 2021 May;249(2):125-134. doi: 10.1530/JOE-20-0437. J Endocrinol. 2021. PMID: 33705349
-
Ultrahigh field diffusion magnetic resonance imaging uncovers intriguing microstructural changes in the adult zebrafish brain caused by Toll-like receptor 2 genomic deletion.NMR Biomed. 2024 Oct;37(10):e5170. doi: 10.1002/nbm.5170. Epub 2024 May 14. NMR Biomed. 2024. PMID: 38742727
-
Metabolomic and transcriptomic profiling of adult mice and larval zebrafish leptin mutants reveal a common pattern of changes in metabolites and signaling pathways.Cell Biosci. 2021 Jul 7;11(1):126. doi: 10.1186/s13578-021-00642-0. Cell Biosci. 2021. PMID: 34233759 Free PMC article.
-
Increased muscle fiber fractional anisotropy value using diffusion tensor imaging after compression without fiber injury.Acta Radiol. 2023 Jan;64(1):139-146. doi: 10.1177/02841851211058282. Epub 2021 Dec 2. Acta Radiol. 2023. PMID: 34854736 Review.
-
Tractography of peripheral nerves and skeletal muscles.Eur J Radiol. 2010 Dec;76(3):391-7. doi: 10.1016/j.ejrad.2010.03.012. Epub 2010 Apr 14. Eur J Radiol. 2010. PMID: 20392583 Review.
Cited by
-
Unveiling the Exquisite Microstructural Details in Zebrafish Brain Non-Invasively Using Magnetic Resonance Imaging at 28.2 T.Molecules. 2024 Sep 29;29(19):4637. doi: 10.3390/molecules29194637. Molecules. 2024. PMID: 39407567 Free PMC article.
References
-
- Zheng BY, He MJ, Jiang J, Luo K, Chen YL, Yan FH. [Construction of leptin gene modified tissue engineered composites in vitro]. Shanghai Kou Qiang Yi Xue. 2016;25(6):641–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

