Ultrahigh-precision noble gas isotope analyses reveal pervasive subsurface fractionation in hydrothermal systems
- PMID: 37058557
- PMCID: PMC10104464
- DOI: 10.1126/sciadv.adg2566
Ultrahigh-precision noble gas isotope analyses reveal pervasive subsurface fractionation in hydrothermal systems
Erratum in
-
Erratum for the Research Article: "Ultrahigh-precision noble gas isotope analyses reveal pervasive subsurface fractionation in hydrothermal systems," by Bekaert et al.Sci Adv. 2024 Feb 23;10(8):eado2859. doi: 10.1126/sciadv.ado2859. Epub 2024 Feb 23. Sci Adv. 2024. PMID: 38394213 Free PMC article. No abstract available.
Abstract
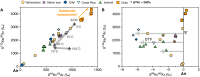
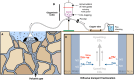
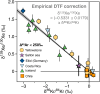
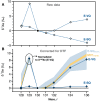
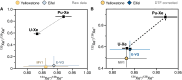
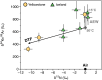
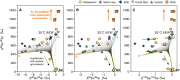
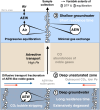
Mantle-derived noble gases in volcanic gases are powerful tracers of terrestrial volatile evolution, as they contain mixtures of both primordial (from Earth's accretion) and secondary (e.g., radiogenic) isotope signals that characterize the composition of deep Earth. However, volcanic gases emitted through subaerial hydrothermal systems also contain contributions from shallow reservoirs (groundwater, crust, atmosphere). Deconvolving deep and shallow source signals is critical for robust interpretations of mantle-derived signals. Here, we use a novel dynamic mass spectrometry technique to measure argon, krypton, and xenon isotopes in volcanic gas with ultrahigh precision. Data from Iceland, Germany, United States (Yellowstone, Salton Sea), Costa Rica, and Chile show that subsurface isotope fractionation within hydrothermal systems is a globally pervasive and previously unrecognized process causing substantial nonradiogenic Ar-Kr-Xe isotope variations. Quantitatively accounting for this process is vital for accurately interpreting mantle-derived volatile (e.g., noble gas and nitrogen) signals, with profound implications for our understanding of terrestrial volatile evolution.
Figures


References
-
- Broadley M. W., Bekaert D. V., Piani L., Füri E., Marty B., Origin of life-forming volatile elements in the inner Solar System. Nature 611, 245–255 (2022). - PubMed
-
- Rubie D. C., Jacobson S. A., Morbidelli A., O’Brien D. P., Young E. D., de Vries J., Nimmo F., Palme H., Frost D. J., Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015).
-
- Bekaert D. V., Turner S. J., Broadley M. W., Barnes J. D., Halldórsson S. A., Labidi J., Wade J., Walowski K. J., Barry P. H., Subduction-driven volatile recycling: A global mass balance. Annu. Rev. Earth Planet. Sci. 49, 37–70 (2021).
-
- Marty B., The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56–66 (2012).
LinkOut - more resources
Full Text Sources
Research Materials

