Aligned Ice Templated Biomaterial Strategies for the Musculoskeletal System
- PMID: 37058583
- PMCID: PMC11468517
- DOI: 10.1002/adhm.202203205
Aligned Ice Templated Biomaterial Strategies for the Musculoskeletal System
Abstract
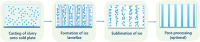
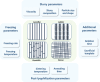
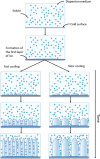
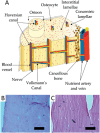
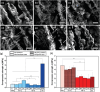
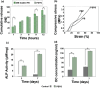
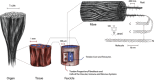
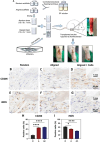
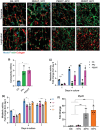
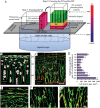
Aligned pore structures present many advantages when conceiving biomaterial strategies for treatment of musculoskeletal disorders. Aligned ice templating (AIT) is one of the many different techniques capable of producing anisotropic porous scaffolds; its high versatility allows for the formation of structures with tunable pore sizes, as well as the use of many different materials. AIT has been found to yield improved compressive properties for bone tissue engineering (BTE), as well as higher tensile strength and optimized cellular alignment and proliferation in tendon and muscle repair applications. This review evaluates the work that has been done in the last decade toward the production of aligned pore structures by AIT with an outlook on the musculoskeletal system. This work describes the fundamentals of the AIT technique and focuses on the research carried out to optimize the biomechanical properties of scaffolds by modifying the pore structure, categorizing by material type and application. Related topics including growth factor incorporation into AIT scaffolds, drug delivery applications, and studies about immune system response will be discussed.
Keywords: aligned scaffolds; biomaterials; bone tissue engineering; musculoskeletal system; unidirectional freezing.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications.Adv Mater. 2021 Aug;33(34):e2100091. doi: 10.1002/adma.202100091. Epub 2021 Jul 8. Adv Mater. 2021. PMID: 34236118 Review.
-
Versatile wedge-based system for the construction of unidirectional collagen scaffolds by directional freezing: practical and theoretical considerations.ACS Appl Mater Interfaces. 2015 Apr 29;7(16):8495-505. doi: 10.1021/acsami.5b00169. Epub 2015 Apr 14. ACS Appl Mater Interfaces. 2015. PMID: 25822583
-
Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying.Acta Biomater. 2019 Aug;94:195-203. doi: 10.1016/j.actbio.2019.05.070. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31154055
-
Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design.J Mech Behav Biomed Mater. 2015 Nov;51:169-83. doi: 10.1016/j.jmbbm.2015.06.033. Epub 2015 Jul 16. J Mech Behav Biomed Mater. 2015. PMID: 26256472
-
A review on directional muscle cell growth in scaffolding biomaterials with aligned porous structures for cultivated meat production.Food Res Int. 2023 Jun;168:112755. doi: 10.1016/j.foodres.2023.112755. Epub 2023 Mar 24. Food Res Int. 2023. PMID: 37120206 Review.
References
-
- Shao G., Hanaor D. A. H., Shen X., Gurlo A., Adv. Mater. 2020, 32, 1907176. - PubMed
-
- Gaudillere C., Serra J. M., Bol. Soc. Esp. Cerám. Vidrio 2016, 55, 45.
-
- Gaudillere C., Garcia‐Fayos J., Serra J. M., J. Mater. Chem. 2014, 2, 3828.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

