Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775
- PMID: 37058687
- PMCID: PMC10414727
- DOI: 10.1200/JCO.22.02152
Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775
Abstract
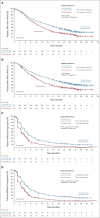
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.We report the final prespecified analysis for overall survival (OS), along with updated progression-free survival (PFS) and objective response rate (ORR), and safety from the open-label, randomized, phase III Study 309/KEYNOTE-775. In total, 827 patients with advanced, recurrent, or metastatic endometrial cancer (EC) were randomly assigned to receive lenvatinib 20 mg orally once daily plus pembrolizumab 200 mg intravenously once every 3 weeks (n = 411) or chemotherapy of the treating physician's choice (doxorubicin 60 mg/m2 intravenously once every 3 weeks or paclitaxel 80 mg/m2 intravenously once weekly [3 weeks on; 1 week off] [n = 416]). Efficacy was reported for patients with mismatch repair proficient (pMMR) tumors and all-comers, and by subgroups (histology, prior therapy, MMR status). Updated safety was also reported.Lenvatinib plus pembrolizumab showed benefits in OS (pMMR HR, 0.70; 95% CI, 0.58 to 0.83; all-comer HR, 0.65; 95% CI, 0.55 to 0.77), PFS (pMMR HR, 0.60; 95% CI, 0.50 to 0.72; all-comer HR, 0.56; 95% CI, 0.48 to 0.66), and ORR (pMMR patients, 32.4% v 15.1%; all-comers, 33.8% v 14.7%) versus chemotherapy. OS, PFS, and ORR favored lenvatinib plus pembrolizumab in all subgroups of interest. No new safety signals were observed. Lenvatinib plus pembrolizumab continued to show improved efficacy versus chemotherapy and manageable safety in patients with previously treated advanced EC.
Trial registration: ClinicalTrials.gov NCT03517449.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Brooks RA, Fleming GF, Lastra RR, et al. : Current recommendations and recent progress in endometrial cancer. CA: Cancer J Clin 69:258-279, 2019 - PubMed
-
- American Cancer Society : Endometrial Cancer Early Detection, Diagnosis, and Staging. https://www.cancer.org/content/dam/CRC/PDF/Public/8611.00.pdf
-
- McMeekin S, Dizon D, Barter J, et al. : Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol 138:18-23, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical