NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson's disease
- PMID: 37058998
- PMCID: PMC10123376
- DOI: 10.1016/j.redox.2023.102698
NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson's disease
Abstract
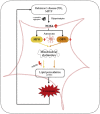
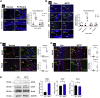
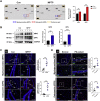
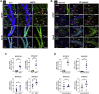
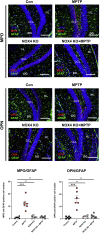
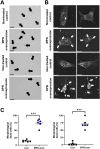
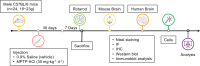
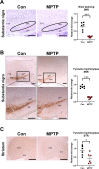
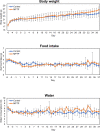
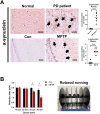
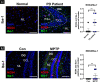
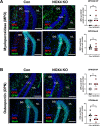
Oxidative stress and mitochondrial dysfunction have been believed to play an important role in the pathogenesis of aging and neurodegenerative diseases, including Parkinson's disease (PD). The excess of reactive oxygen species (ROS) increases with age and causes a redox imbalance, which contributes to the neurotoxicity of PD. Accumulating evidence suggests that NADPH oxidase (NOX)-derived ROS, especially NOX4, belong to the NOX family and is one of the major isoforms expressed in the central nervous system (CNS), associated with the progression of PD. We have previously shown that NOX4 activation regulates ferroptosis via astrocytic mitochondrial dysfunction. We have previously shown that activation of NOX4 regulates ferroptosis through mitochondrial dysfunction in astrocytes. However, it remains unclear why an increase in NOX4 in neurodegenerative diseases leads to astrocyte cell death by certain mediators. Therefore, this study was designed to evaluate how NOX4 in the hippocampus is involved in PD by comparing an MPTP-induced PD mouse model compared to human PD patients. We could detect that the hippocampus was dominantly associated with elevated levels of NOX4 and α-synuclein during PD and the neuroinflammatory cytokines, myeloperoxidase (MPO) and osteopontin (OPN), were upregulated particularly in astrocytes. Intriguingly, NOX4 suggested a direct intercorrelation with MPO and OPN in the hippocampus. Upregulation of MPO and OPN induces mitochondrial dysfunction by suppressing five protein complexes in the mitochondrial electron transport system (ETC) and increases the level of 4-HNE leading to ferroptosis in human astrocytes. Overall, our findings indicate that the elevation of NOX4 cooperated with the MPO and OPN inflammatory cytokines through mitochondrial aberration in hippocampal astrocytes during PD.
Keywords: Hippocampus; Mitochondria; Myeloperoxidase; NADPH oxidase 4 (NOX4); Osteopontin; Parkinson's disease.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Aarsland D., Batzu L., Halliday G.M., Geurtsen G.J., Ballard C., Ray Chaudhuri K., Weintraub D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021;7(1):47. - PubMed
-
- Wright R. Mitochondrial dysfunction and Parkinson's disease. Nat. Neurosci. 2022;25(1):2. - PubMed
-
- Winklhofer K.F., Haass C. Mitochondrial dysfunction in Parkinson's disease. Biochim. Biophys. Acta. 2010;1802(1):29–44. - PubMed
Further reading
-
- Davies M.J., Hawkins C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxidants Redox Signal. 2020;32(13):957–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

