Metastasis
- PMID: 37059065
- PMCID: PMC10511214
- DOI: 10.1016/j.cell.2023.03.003
Metastasis
Abstract
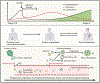
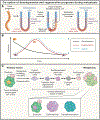
Most cancer-associated deaths occur due to metastasis, yet our understanding of metastasis as an evolving, heterogeneous, systemic disease and of how to effectively treat it is still emerging. Metastasis requires the acquisition of a succession of traits to disseminate, variably enter and exit dormancy, and colonize distant organs. The success of these events is driven by clonal selection, the potential of metastatic cells to dynamically transition into distinct states, and their ability to co-opt the immune environment. Here, we review the main principles of metastasis and highlight emerging opportunities to develop more effective therapies for metastatic cancer.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.G. is an inventor on patents related to targeting metastatic cancer.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

