Fibroblasts in cancer: Unity in heterogeneity
- PMID: 37059066
- PMCID: PMC11422789
- DOI: 10.1016/j.cell.2023.03.016
Fibroblasts in cancer: Unity in heterogeneity
Abstract
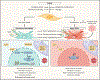
Tumor cells do not exist in isolation in vivo, and carcinogenesis depends on the surrounding tumor microenvironment (TME), composed of a myriad of cell types and biophysical and biochemical components. Fibroblasts are integral in maintaining tissue homeostasis. However, even before a tumor develops, pro-tumorigenic fibroblasts in close proximity can provide the fertile 'soil' to the cancer 'seed' and are known as cancer-associated fibroblasts (CAFs). In response to intrinsic and extrinsic stressors, CAFs reorganize the TME enabling metastasis, therapeutic resistance, dormancy and reactivation by secreting cellular and acellular factors. In this review, we summarize the recent discoveries on CAF-mediated cancer progression with a particular focus on fibroblast heterogeneity and plasticity.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interest.
Figures




References
-
- Kochetkova M, and Samuel MS (2022). Differentiation of the tumor microenvironment: are CAFs the Organizer? Trends Cell Biol. 32, 285–294. - PubMed
-
- Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, Jeng YM, Chang KJ, Lee EYHP, Shew JY, and Lee WH (2012). Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res. 72, 4652–4661. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

