The role of extracellular vesicles in cancer
- PMID: 37059067
- PMCID: PMC10484374
- DOI: 10.1016/j.cell.2023.03.010
The role of extracellular vesicles in cancer
Abstract
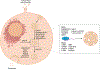
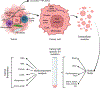
Intercellular communication is a key feature of cancer progression and metastasis. Extracellular vesicles (EVs) are generated by all cells, including cancer cells, and recent studies have identified EVs as key mediators of cell-cell communication via packaging and transfer of bioactive constituents to impact the biology and function of cancer cells and cells of the tumor microenvironment. Here, we review recent advances in understanding the functional contribution of EVs to cancer progression and metastasis, as cancer biomarkers, and the development of cancer therapeutics.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests MD Anderson Cancer Center and R.K. hold patents in the area of exosome biology and are licensed to Codiak Biosciences, Inc. MD Anderson Cancer Center and R.K. are stock equity holders in Codiak Biosciences, Inc. R.K. is a consultant and scientific adviser for Codiak Biosciences, Inc.
Figures



References
-
- Johnstone RM, Adam M, Hammond JR, Orr L, and Turbide C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262, 9412–9420. - PubMed
-
- Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, Zanivan S, and Kalluri R. (2021). Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol 23, 631–641. 10.1038/s41556-021-00693-y. - DOI - PMC - PubMed
-
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, and Thery C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 113, E968–977. 10.1073/pnas.1521230113. - DOI - PMC - PubMed
-
- Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, et al. (2021). Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 12, 4389. 10.1038/s41467-021-24384-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

