Biomarkers Found in the Tumor Interstitial Fluid may Help Explain the Differential Behavior Among Keratinocyte Carcinomas
- PMID: 37059366
- PMCID: PMC10203780
- DOI: 10.1016/j.mcpro.2023.100547
Biomarkers Found in the Tumor Interstitial Fluid may Help Explain the Differential Behavior Among Keratinocyte Carcinomas
Abstract
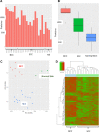
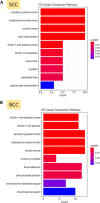
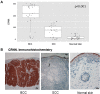
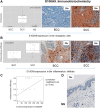
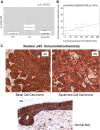
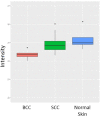
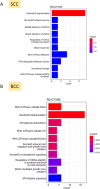
Basal cell carcinomas (BCCs) and cutaneous squamous cell carcinomas (SCCs) are the most frequent types of cancer, and both originate from the keratinocyte transformation, giving rise to the group of tumors called keratinocyte carcinomas (KCs). The invasive behavior is different in each group of KC and may be influenced by their tumor microenvironment. The principal aim of the study is to characterize the protein profile of the tumor interstitial fluid (TIF) of KC to evaluate changes in the microenvironment that could be associated with their different invasive and metastatic capabilities. We obtained TIF from 27 skin biopsies and conducted a label-free quantitative proteomic analysis comparing seven BCCs, 16 SCCs, and four normal skins. A total of 2945 proteins were identified, 511 of them quantified in more than half of the samples of each tumoral type. The proteomic analysis revealed differentially expressed TIF proteins that could explain the different metastatic behavior in both KCs. In detail, the SCC samples disclosed an enrichment of proteins related to cytoskeleton, such as Stratafin and Ladinin-1. Previous studies found their upregulation positively correlated with tumor progression. Furthermore, the TIF of SCC samples was enriched with the cytokines S100A8/S100A9. These cytokines influence the metastatic output in other tumors through the activation of NF-kB signaling. According to this, we observed a significant increase in nuclear NF-kB subunit p65 in SCCs but not in BCCs. In addition, the TIF of both tumors was enriched with proteins involved in the immune response, highlighting the relevance of this process in the composition of the tumor environment. Thus, the comparison of the TIF composition of both KCs provides the discovery of a new set of differential biomarkers. Among them, secreted cytokines such as S100A9 may help explain the higher aggressiveness of SCCs, while Cornulin is a specific biomarker for BCCs. Finally, the proteomic landscape of TIF provides key information on tumor growth and metastasis, which can contribute to the identification of clinically applicable biomarkers that may be used in the diagnosis of KC, as well as therapeutic targets.
Keywords: S100A9; basal cell carcinoma; keratinocyte carcinomas; proteomics; squamous cell carcinoma; tumor interstitial fluid.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest to disclosure.
Figures





References
-
- Matas-Nadal C., Sagristà M., Gómez-Arbonés X., Sobrino Bermejo C., Fernández-Armenteros J.M., Àngel Baldó J., et al. Risk factors for early-onset basal cell carcinomas and the trend towards their female predominance. J. Dtsch. Dermatol. Ges. 2021;19:364–371. - PubMed
-
- Ionescu D.N., Arida M., Jukic D.M. Metastatic basal cell carcinoma: four case reports, review of literature, and immunohistochemical evaluation. Arch. Pathol. Lab. Med. 2006;130:45–51. - PubMed
-
- Burian M., Velic A., Matic K., Günther S., Kraft B., Gonser L., et al. Quantitative proteomics of the human skin secretome reveal a reduction in immune defense mediators in ectodermal dysplasia patients. J. Invest. Dermatol. 2015;135:759–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

