Specific behaviors during auditory fear conditioning and postsynaptic expression of AMPA receptors in the basolateral amygdala predict interindividual differences in fear generalization in male rats
- PMID: 37059464
- PMCID: PMC10165993
- DOI: 10.1101/lm.053612.122
Specific behaviors during auditory fear conditioning and postsynaptic expression of AMPA receptors in the basolateral amygdala predict interindividual differences in fear generalization in male rats
Abstract
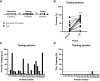
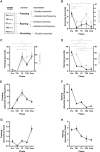
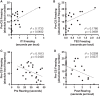
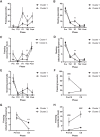
Auditory fear conditioning in rats is a widely used method to study learning, memory, and emotional responding. Despite procedural standardizations and optimizations, there is substantial interindividual variability in fear expression during test, notably in terms of fear expressed toward the testing context alone. To better understand which factors could explain this variation between subjects, we here explored whether behavior during training and expression of AMPA receptors (AMPARs) after long-term memory formation in the amygdala could predict freezing during test. We studied outbred male rats and found strong variation in fear generalization to a different context. Hierarchical clustering of these data identified two distinct groups of subjects that independently correlated with a specific pattern of behaviors expressed during initial training (i.e., rearing and freezing). The extent of fear generalization correlated positively with postsynaptic expression of GluA1-containing AMPA receptors in the basolateral nucleus of the amygdala. Our data thus identify candidate behavioral and molecular predictors of fear generalization that may inform our understanding of some anxiety-related disorders, such as posttraumatic stress disorder (PTSD), that are characterized by overgeneralized fear.
© 2023 Moraes and Hardt; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
Increased Fear Generalization and Amygdala AMPA Receptor Proteins in Chronic Traumatic Brain Injury.J Neurotrauma. 2022 Nov;39(21-22):1561-1574. doi: 10.1089/neu.2022.0119. Epub 2022 Sep 9. J Neurotrauma. 2022. PMID: 35722903 Free PMC article.
-
NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats.Eur J Neurosci. 2010 May;31(9):1664-70. doi: 10.1111/j.1460-9568.2010.07223.x. Eur J Neurosci. 2010. PMID: 20525079 Free PMC article.
-
Prior stress promotes the generalization of contextual fear memories: Involvement of the gabaergic signaling within the basolateral amygdala complex.Prog Neuropsychopharmacol Biol Psychiatry. 2018 Apr 20;83:18-26. doi: 10.1016/j.pnpbp.2017.12.003. Epub 2017 Dec 7. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 29223783
-
Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress.Neuropsychopharmacology. 2016 Jan;41(1):45-57. doi: 10.1038/npp.2015.224. Epub 2015 Aug 6. Neuropsychopharmacology. 2016. PMID: 26329286 Free PMC article. Review.
-
Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits.Neuroscience. 2013 Sep 5;247:253-72. doi: 10.1016/j.neuroscience.2013.05.050. Epub 2013 Jun 1. Neuroscience. 2013. PMID: 23732229 Review.
Cited by
-
Behavioral and genetic architecture of fear conditioning and related phenotypes.Neurobiol Learn Mem. 2023 Nov;205:107837. doi: 10.1016/j.nlm.2023.107837. Epub 2023 Oct 5. Neurobiol Learn Mem. 2023. PMID: 37805118 Free PMC article.
References
-
- Bhattacharya S, Kimble W, Buabeid M, Bhattacharya D, Bloemer J, Alhowail A, Reed M, Dhanasekaran M, Escobar M, Suppiramaniam V. 2017. Altered AMPA receptor expression plays an important role in inducing bidirectional synaptic plasticity during contextual fear memory reconsolidation. Neurobiol Learn Mem 139: 98–108. 10.1016/j.nlm.2016.12.013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
