Rational Design of the β-Bulge Gate in a Green Fluorescent Protein Accelerates the Kinetics of Sulfate Sensing
- PMID: 37059690
- PMCID: PMC10330437
- DOI: 10.1002/anie.202302304
Rational Design of the β-Bulge Gate in a Green Fluorescent Protein Accelerates the Kinetics of Sulfate Sensing
Abstract
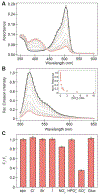
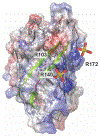
Detection of anions in complex aqueous media is a fundamental challenge with practical utility that can be addressed by supramolecular chemistry. Biomolecular hosts such as proteins can be used and adapted as an alternative to synthetic hosts. Here, we report how the mutagenesis of the β-bulge residues (D137 and W138) in mNeonGreen, a bright, monomeric fluorescent protein, unlocks and tunes the anion preference at physiological pH for sulfate, resulting in the turn-off sensor SulfOFF-1. This unprecedented sensing arises from an enhancement in the kinetics of binding, largely driven by position 138. In line with these data, molecular dynamics (MD) simulations capture how the coordinated entry and gating of sulfate into the β-barrel is eliminated upon mutagenesis to facilitate binding and fluorescence quenching.
Keywords: Anions; Molecular Dynamics; Protein Engineering; Sensors; Supramolecular Chemistry.
© 2023 Wiley-VCH GmbH.
Figures



Similar articles
-
Genetically encoded green fluorescent sensor for probing sulfate transport activity of solute carrier family 26 member a2 (Slc26a2) protein.Commun Biol. 2024 Oct 23;7(1):1375. doi: 10.1038/s42003-024-07020-9. Commun Biol. 2024. PMID: 39443638 Free PMC article.
-
Identification of mNeonGreen as a pH-Dependent, Turn-On Fluorescent Protein Sensor for Chloride.Chembiochem. 2019 Jul 15;20(14):1759-1765. doi: 10.1002/cbic.201900147. Epub 2019 May 8. Chembiochem. 2019. PMID: 30843313 Free PMC article.
-
Novel Fluorescence Guanidine Molecules for Selective Sulfate Anion Detection in Water Complex Samples over a Wide pH Range.ACS Sens. 2021 Sep 24;6(9):3224-3233. doi: 10.1021/acssensors.1c00876. Epub 2021 Aug 31. ACS Sens. 2021. PMID: 34464091
-
Alfred Werner revisited: the coordination chemistry of anions.Acc Chem Res. 2005 Aug;38(8):671-8. doi: 10.1021/ar040071t. Acc Chem Res. 2005. PMID: 16104690 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
NitrOFF: An engineered fluorescent biosensor to illuminate nitrate transport in living cells.bioRxiv [Preprint]. 2025 Mar 23:2025.03.22.644677. doi: 10.1101/2025.03.22.644677. bioRxiv. 2025. PMID: 40166251 Free PMC article. Preprint.
-
A chemigenetic indicator based on a synthetic chelator and a green fluorescent protein for imaging of intracellular sodium ions.RSC Chem Biol. 2024 Dec 3;6(2):170-174. doi: 10.1039/d4cb00256c. eCollection 2025 Feb 5. RSC Chem Biol. 2024. PMID: 39678364 Free PMC article.
-
Genetically encoded green fluorescent sensor for probing sulfate transport activity of solute carrier family 26 member a2 (Slc26a2) protein.Commun Biol. 2024 Oct 23;7(1):1375. doi: 10.1038/s42003-024-07020-9. Commun Biol. 2024. PMID: 39443638 Free PMC article.
-
Engineering the ChlorON Series: Turn-On Fluorescent Protein Sensors for Imaging Labile Chloride in Living Cells.ACS Cent Sci. 2023 Dec 18;10(1):77-86. doi: 10.1021/acscentsci.3c01088. eCollection 2024 Jan 24. ACS Cent Sci. 2023. PMID: 38292617 Free PMC article.
-
Illuminating anions in biology with genetically encoded fluorescent biosensors.Curr Opin Chem Biol. 2025 Feb;84:102548. doi: 10.1016/j.cbpa.2024.102548. Epub 2024 Dec 9. Curr Opin Chem Biol. 2025. PMID: 39657518 Review.
References
-
- Wang Y-Y, Cheng Y-H, Chen K-E, Tsay Y-F, Annu. Rev. Plant Biol 2018, 69, 85–122. - PubMed
-
- Yin YB, Guo S, Heck KN, Clark CA, Conrad CL, Wong MS, ACS Sustain. Chem. Eng 2018, 6, 11160–11175.
-
- Zajac M, Chakraborty K, Saha S, Mahadevan V, Infield DT, Accardi A, Qiu Z, Krishnan Y, J. Cell Sci 2020, 133, jcs240390. - PubMed
-
- Busschaert N, Caltagirone C, Van Rossom W, Gale PA, Chem. Rev 2015, 115, 8038–8155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

