Intermediate hepatitis B virus infection prevalence among 1622 pregnant women in rural Burkina Faso and implications for mother-to-child transmission
- PMID: 37059812
- PMCID: PMC10103033
- DOI: 10.1038/s41598-023-32766-3
Intermediate hepatitis B virus infection prevalence among 1622 pregnant women in rural Burkina Faso and implications for mother-to-child transmission
Abstract
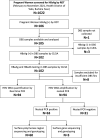
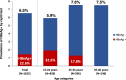
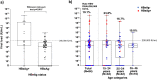
In highly endemic countries for hepatitis B virus (HBV) infection, childhood infection, including mother-to-child transmission (MTCT), represents the primary transmission route. High maternal DNA level (viral load ≥ 200,000 IU/mL) is a significant factor for MTCT. We investigated the prevalence of HBsAg, HBeAg, and high HBV DNA among pregnant women in three hospitals in Burkina Faso and assessed the performance of HBeAg to predict high viral load. Consenting pregnant women were interviewed on their sociodemographic characteristics and tested for HBsAg by a rapid diagnostic test, and dried blood spot (DBS) samples were collected for laboratory analyses. Of the 1622 participants, HBsAg prevalence was 6.5% (95% CI, 5.4-7.8%). Among 102 HBsAg-positive pregnant women in DBS samples, HBeAg was positive in 22.6% (95% CI, 14.9-31.9%), and viral load was quantified in 94 cases, with 19.1% having HBV DNA ≥ 200,000 IU/mL. HBV genotypes were identified in 63 samples and predominant genotypes were E (58.7%) and A (36.5%). The sensitivity of HBeAg by using DBS samples to identify high viral load in the 94 cases was 55.6%, and the specificity was 86.8%. These findings highlight the need to implement routine HBV screening and effective MTCT risk assessment for all pregnant women in Burkina Faso to enable early interventions that can effectively reduce MTCT.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Published online, 108 (2021).
-
- World Health Organization. Interim Guidance For Country Validation of Viral Hepatitis Elimination. 1–96 (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

