Activated CD90/Thy-1 fibroblasts co-express the Δ133p53β isoform and are associated with highly inflamed rheumatoid arthritis
- PMID: 37060003
- PMCID: PMC10105423
- DOI: 10.1186/s13075-023-03040-8
Activated CD90/Thy-1 fibroblasts co-express the Δ133p53β isoform and are associated with highly inflamed rheumatoid arthritis
Abstract
Background: The p53 isoform Δ133p53β is known to be associated with cancers driven by inflammation. Many of the features associated with the development of inflammation in rheumatoid arthritis (RA) parallel those evident in cancer progression. However, the role of this isoform in RA has not yet been explored. The aim of this study was to determine whether Δ133p53β is driving aggressive disease in RA.
Methods: Using RA patient synovia, we carried out RT-qPCR and RNAScope-ISH to determine both protein and mRNA levels of Δ133p53 and p53. We also used IHC to determine the location and type of cells with elevated levels of Δ133p53β. Plasma cytokines were also measured using a BioPlex cytokine panel and data analysed by the Milliplex Analyst software.
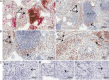
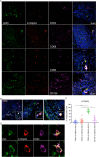
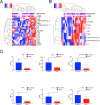
Results: Elevated levels of pro-inflammatory plasma cytokines were associated with synovia from RA patients displaying extensive tissue inflammation, increased immune cell infiltration and the highest levels of Δ133TP53 and TP53β mRNA. Located in perivascular regions of synovial sub-lining and surrounding ectopic lymphoid structures (ELS) were a subset of cells with high levels of CD90, a marker of 'activated fibroblasts' together with elevated levels of Δ133p53β.
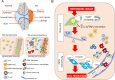
Conclusions: Induction of Δ133p53β in CD90+ synovial fibroblasts leads to an increase in cytokine and chemokine expression and the recruitment of proinflammatory cells into the synovial joint, creating a persistently inflamed environment. Our results show that dysregulated expression of Δ133p53β could represent one of the early triggers in the immunopathogenesis of RA and actively perpetuates chronic synovial inflammation. Therefore, Δ133p53β could be used as a biomarker to identify RA patients more likely to develop aggressive disease who might benefit from targeted therapy to cytokines such as IL-6.
Keywords: CD90; Fibroblasts; Inflammation; Rheumatoid arthritis; Synoviocytes; p53 isoforms.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ai R, Laragione T, Hammaker D, Boyle DL, Wildberg A, Maeshima K, Palescandolo E, Krishna V, Pocalyko D, Whitaker JW, Bai Y, Nagpal S, Bachman KE, Ainsworth RI, Wang M, Ding B, Gulko PS, Wang W, Firestein GS. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018;9(1):1921. doi: 10.1038/s41467-018-04310-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

