Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol)
- PMID: 37060055
- PMCID: PMC10105392
- DOI: 10.1186/s12885-023-10829-y
Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol)
Abstract
Background: Despite standard treatments including chemoradiotherapy with temozolomide (TMZ) (STUPP protocol), the prognosis of glioblastoma patients remains poor. AGuIX nanoparticles have a high radiosensitizing potential, a selective and long-lasting accumulation in tumors and a rapid renal elimination. Their therapeutic effect has been proven in vivo on several tumor models, including glioblastoma with a potential synergetic effect when combined with TMZ based chemoradiotherapy, and they are currently evaluated in 4 ongoing Phase Ib and II clinical trials in 4 indications (brain metastases, lung, pancreatic and cervix cancers) (> 100 patients received AGuIX). Thus, they could offer new perspectives for patients with newly diagnosed glioblastoma. The aim of this study is to determine the recommended dose of AGuIX as a radiosensitizer in combination with radiotherapy and TMZ during the concurrent radio-chemotherapy period for phase II (RP2D) and to estimate the efficacy of the combination.
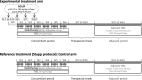
Methods: NANO-GBM is a multicenter, phase I/II, randomized, open-label, non-comparative, therapeutic trial. According to a dose escalation scheme driven by a TITE-CRM design, 3 dose levels of AGuIX (50, 75 and 100 mg/kg) will be tested in phase I added to standard concomitant radio-chemotherapy. Patients with grade IV glioblastoma, not operated or partially operated, with a KPS ≥ 70% will be eligible for the study. The primary endpoints are i) for phase I, the RP2D of AGuIX, with DLT defined as any grade 3-4 NCI-CTCAE toxicity and ii) for phase II, the 6-month progression-free survival rate. The pharmacokinetics, distribution of nanoparticles, tolerance of the combination, neurological status, overall survival (median, 6-month and 12-month rates), response to treatment, and progression-free survival (median and 12-month rates) will be assessed as secondary objectives. Maximum sixty-six patients are expected to be recruited in the study from 6 sites.
Discussion: The use of AGuIX nanoparticles could allow to overpass the radioresistance to the reference treatment of newly diagnosed glioblastomas that have the poorest prognosis (incomplete resection or biopsy only).
Trial registration: Clinicaltrials.gov: NCT04881032 , registered on April 30, 2021. Identifier with the French National Agency for the Safety of Medicines and Health Products (ANSM): N°Eudra CT 2020-004552-15.
Protocol: version 3, 23 May 2022.
Keywords: AGuIX; Glioblastoma; Nanomedicine; Nanoparticles; Radiosensitization; Radiotherapy.
© 2023. The Author(s).
Conflict of interest statement
GLD discloses patent No. WO2009/053644 which protects the AGuIX nanoparticles described in this publication. SD, KS, ML, ODB and GLD are employees of NH TherAguix (Meylan, France), which is developing the AGuIX nanoparticles. SD, ML and GLD own shares in this company. The other authors declare that they have no competing interests.
Figures
Similar articles
-
Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol.BMC Cancer. 2019 Mar 4;19(1):198. doi: 10.1186/s12885-019-5413-y. BMC Cancer. 2019. PMID: 30832617 Free PMC article.
-
NANO-GBM trial of AGuIX nanoparticles with radiotherapy and temozolomide in the treatment of newly diagnosed Glioblastoma: Phase 1b outcomes and MRI-based biodistribution.Clin Transl Radiat Oncol. 2024 Jul 31;48:100833. doi: 10.1016/j.ctro.2024.100833. eCollection 2024 Sep. Clin Transl Radiat Oncol. 2024. PMID: 39184998 Free PMC article.
-
A randomised phase II trial of temozolomide with or without cannabinoids in patients with recurrent glioblastoma (ARISTOCRAT): protocol for a multi-centre, double-blind, placebo-controlled trial.BMC Cancer. 2024 Jan 15;24(1):83. doi: 10.1186/s12885-023-11792-4. BMC Cancer. 2024. PMID: 38225549 Free PMC article.
-
Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience.J Neurooncol. 2012 Apr;107(2):395-405. doi: 10.1007/s11060-011-0766-3. Epub 2011 Nov 22. J Neurooncol. 2012. PMID: 22105851 Review.
-
Therapeutic approach beyond conventional temozolomide for newly diagnosed glioblastoma: Review of the present evidence and future direction.Indian J Med Paediatr Oncol. 2015 Oct-Dec;36(4):229-37. doi: 10.4103/0971-5851.171543. Indian J Med Paediatr Oncol. 2015. PMID: 26811592 Free PMC article. Review.
Cited by
-
Targeted-theranostic nanoparticles induce anti-tumor immune response in lung cancer.J Nanobiotechnology. 2025 Jul 1;23(1):466. doi: 10.1186/s12951-025-03542-4. J Nanobiotechnology. 2025. PMID: 40598513 Free PMC article.
-
Particle Beam Radiobiology Status and Challenges: A PTCOG Radiobiology Subcommittee Report.Int J Part Ther. 2024 Aug 8;13:100626. doi: 10.1016/j.ijpt.2024.100626. eCollection 2024 Sep. Int J Part Ther. 2024. PMID: 39258166 Free PMC article. Review.
-
Second-Generation Gadolinium-Bismuth Ultrasmall Nanoparticles Amplify the Effects of Clinical Radiation Therapy and Provide Clinical Magnetic Resonance Imaging Contrast.Int J Radiat Oncol Biol Phys. 2025 May 8:S0360-3016(25)00420-1. doi: 10.1016/j.ijrobp.2025.04.032. Online ahead of print. Int J Radiat Oncol Biol Phys. 2025. PMID: 40348199
-
Genomic predictors of radiation response: recent progress towards personalized radiotherapy for brain metastases.Cell Death Discov. 2024 Dec 18;10(1):501. doi: 10.1038/s41420-024-02270-2. Cell Death Discov. 2024. PMID: 39695143 Free PMC article. Review.
-
Brain-targeting drug delivery systems: The state of the art in treatment of glioblastoma.Mater Today Bio. 2025 Jan 3;30:101443. doi: 10.1016/j.mtbio.2025.101443. eCollection 2025 Feb. Mater Today Bio. 2025. PMID: 39866779 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, Bozzao A, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2010;97(3):377–81. doi: 10.1016/j.radonc.2010.08.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous