A Phase I, First-in-Human Study of PRL3-zumab in Advanced, Refractory Solid Tumors and Hematological Malignancies
- PMID: 37060431
- PMCID: PMC10192144
- DOI: 10.1007/s11523-023-00962-w
A Phase I, First-in-Human Study of PRL3-zumab in Advanced, Refractory Solid Tumors and Hematological Malignancies
Abstract
Background: Phosphatase of regenerating liver-3 (PRL-3) is involved in cellular processes driving metastasis, cell proliferation, invasion, motility and survival. It has been shown to be upregulated and overexpressed in cancer tissue, in contrast to low or no expression in most normal tissue. PRL3-zumab is a first-in-class humanized antibody that specifically binds to PRL-3 oncotarget with high affinity and has been shown to reduce tumor growth and increase survival.
Objective: In the study, we aimed to determine the safety and efficacy of PRL3-zumab in patients with advanced solid tumors and hematological malignancies.
Methods: We conducted a phase I, first-in-human study in advanced solid tumors and hematological malignancies to investigate the safety, tolerability and efficacy of PRL3-zumab. Response rates were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1) for solid tumors. For acute myeloid leukemia (AML) patients, bone marrow response criteria based on the European Leukaemia Network (ELN) 2017 guidelines for AML were used. We also explored the pharmacokinetics and pharmacodynamic relationships of PRL3-zumab in patients. This study was registered with ClinicalTrials.gov: NCT03191682.
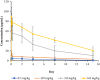
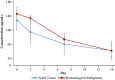
Results: In the dose-escalation cohort, 11 patients with advanced solid tumors were enrolled into the study. An additional 12 patients with solid tumors and four patients with AML were enrolled in the dose-expansion cohort. Maximum tolerability was not achieved in this study, as there were no dose-limiting toxicities. Potential treatment-emergent adverse events were grade 1 increased stoma output and fatigue and grade 2 vomiting. Best response observed was stable disease in three solid-tumor patients (11.1%). The pharmacokinetics of PRL3-zumab were dose proportional, consistent with an IgG type monoclonal antibody.
Conclusions: PRL3-zumab, a first-in-class humanized antibody, was safe and tolerable in solid tumors and hematological malignancies.
© 2023. The Author(s).
Conflict of interest statement
Qi Zeng is the founder of Intra-ImmuSG Pte. Ltd (IISG), an A*STAR spin-off company that provided PRL3-zumab for this study. Cheng E. Chee, Melissa Ooi, Soo-Chin Lee, Raghav Sundar, Valerie Heong, Wei-Peng Yong, Chin Hin Ng, Andrea Wong, Joline SJ Lim, David SP Tan, Ross Soo, Joshua TC Tan, Song Yang, Min Thura, Abdul Qader Al-Aidaroos, Wee Joo Chng and Boon-Cher Goh declare that they have no conflicts of interest that might be relevant to the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

