Pathological angiogenesis: mechanisms and therapeutic strategies
- PMID: 37060495
- PMCID: PMC10105163
- DOI: 10.1007/s10456-023-09876-7
Pathological angiogenesis: mechanisms and therapeutic strategies
Abstract
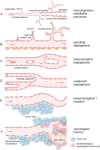
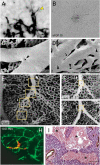
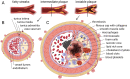
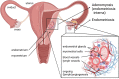
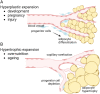
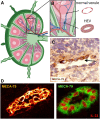
In multicellular organisms, angiogenesis, the formation of new blood vessels from pre-existing ones, is an essential process for growth and development. Different mechanisms such as vasculogenesis, sprouting, intussusceptive, and coalescent angiogenesis, as well as vessel co-option, vasculogenic mimicry and lymphangiogenesis, underlie the formation of new vasculature. In many pathological conditions, such as cancer, atherosclerosis, arthritis, psoriasis, endometriosis, obesity and SARS-CoV-2(COVID-19), developmental angiogenic processes are recapitulated, but are often done so without the normal feedback mechanisms that regulate the ordinary spatial and temporal patterns of blood vessel formation. Thus, pathological angiogenesis presents new challenges yet new opportunities for the design of vascular-directed therapies. Here, we provide an overview of recent insights into blood vessel development and highlight novel therapeutic strategies that promote or inhibit the process of angiogenesis to stabilize, reverse, or even halt disease progression. In our review, we will also explore several additional aspects (the angiogenic switch, hypoxia, angiocrine signals, endothelial plasticity, vessel normalization, and endothelial cell anergy) that operate in parallel to canonical angiogenesis mechanisms and speculate how these processes may also be targeted with anti-angiogenic or vascular-directed therapies.
Keywords: Angiogenesis; Anti-angiogenesis; Endothelial cells; Immunotherapy; Vascular biology; Vascular targeting.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52(2):237–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

