VE303, a Defined Bacterial Consortium, for Prevention of Recurrent Clostridioides difficile Infection: A Randomized Clinical Trial
- PMID: 37060545
- PMCID: PMC10105904
- DOI: 10.1001/jama.2023.4314
VE303, a Defined Bacterial Consortium, for Prevention of Recurrent Clostridioides difficile Infection: A Randomized Clinical Trial
Abstract
Importance: The effect of rationally defined nonpathogenic, nontoxigenic, commensal strains of Clostridia on prevention of Clostridioides difficile infection (CDI) is unknown.
Objective: To determine the efficacy of VE303, a defined bacterial consortium of 8 strains of commensal Clostridia, in adults at high risk for CDI recurrence. The primary objective was to determine the recommended VE303 dosing for a phase 3 trial.
Design, setting, and participants: Phase 2, randomized, double-blind, placebo-controlled, dose-ranging study conducted from February 2019 to September 2021 at 27 sites in the US and Canada. The study included 79 participants aged 18 years or older who were diagnosed with laboratory-confirmed CDI with 1 or more prior CDI episodes in the last 6 months and those with primary CDI at high risk for recurrence (defined as aged ≥75 years or ≥65 years with ≥1 risk factors: creatinine clearance <60 mL/min/1.73 m2, proton pump inhibitor use, remote [>6 months earlier] CDI history).
Interventions: Participants were randomly assigned to high-dose VE303 (8.0 × 109 colony-forming units [CFUs]) (n = 30), low-dose VE303 (1.6 × 109 CFUs) (n = 27), or placebo capsules (n = 22) orally once daily for 14 days.
Main outcomes and measures: The primary efficacy end point was the proportion of participants with CDI recurrence at 8 weeks using a combined clinical and laboratory definition. The primary efficacy end point was analyzed in 3 prespecified analyses, using successively broader definitions for an on-study CDI recurrence: (1) diarrhea consistent with CDI plus a toxin-positive stool sample; (2) diarrhea consistent with CDI plus a toxin-positive, polymerase chain reaction-positive, or toxigenic culture-positive stool sample; and (3) diarrhea consistent with CDI plus laboratory confirmation or (in the absence of a stool sample) treatment with a CDI-targeted antibiotic.
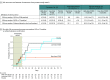
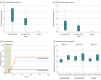
Results: Baseline characteristics were similar across the high-dose VE303 (n = 29; 1 additional participant excluded from efficacy analysis), low-dose VE303 (n = 27), and placebo (n = 22) groups. The participants' median age was 63.5 years (range, 24-96); 70.5% were female; and 1.3% were Asian, 1.3% Black, 2.6% Hispanic, and 96.2% White. CDI recurrence rates through week 8 (using the efficacy analysis 3 definition) were 13.8% (4/29) for high-dose VE303, 37.0% (10/27) for low-dose VE303, and 45.5% (10/22) for placebo (P = .006, high-dose VE303 vs placebo).
Conclusions and relevance: Among adults with laboratory-confirmed CDI with 1 or more prior CDI episodes in the last 6 months and those with primary CDI at high risk for recurrence, high-dose VE303 prevented recurrent CDI compared with placebo. A larger, phase 3 study is needed to confirm these findings.
Trial registration: ClinicalTrials.gov Identifier: NCT03788434.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

