Immunity to pathogenic fungi in the eye
- PMID: 37060806
- PMCID: PMC10508057
- DOI: 10.1016/j.smim.2023.101753
Immunity to pathogenic fungi in the eye
Abstract
Fusarium, Aspergillus and Candida are important fungal pathogens that cause visual impairment and blindness in the USA and worldwide. This review will summarize the epidemiology and clinical features of corneal infections and discuss the immune and inflammatory responses that play an important role in clinical disease. In addition, we describe fungal virulence factors that are required for survival in infected corneas, and the activities of neutrophils in fungal killing, tissue damage and cytokine production.
Keywords: Aspergillus; Candida; Cornea; Cytokines; Fungal infection; Fusarium; IL-1 alpha; IL-1 beta; Innate immunity; Keratitis; NADPH oxidase; Neutrophil; Nutritional immunity; Ocular infection; Reactive oxygen species.
Copyright © 2023. Published by Elsevier Ltd.
Figures


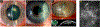
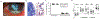


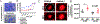


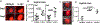

References
-
- Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW, The global incidence and diagnosis of fungal keratitis, Lancet Infect. Dis 21 (3) (2021) e49–e57. - PubMed
-
- Mills B, Radhakrishnan N, Rajapandian S.G. Karthikeyan, Rameshkumar G, Lalitha P, Prajna NV, The role of fungi in fungal keratitis, Exp. Eye Res 202 (2021), 108372. - PubMed

