Aberrant differentiation of second heart field mesoderm prefigures cellular defects in the outflow tract in response to loss of FGF8
- PMID: 37060937
- PMCID: PMC10686765
- DOI: 10.1016/j.ydbio.2023.04.001
Aberrant differentiation of second heart field mesoderm prefigures cellular defects in the outflow tract in response to loss of FGF8
Abstract
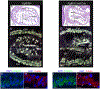
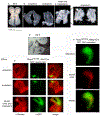
Development of the outflow tract of the heart requires specification, proliferation and deployment of a progenitor cell population from the second heart field to generate the myocardium at the arterial pole of the heart. Disruption of these processes leads to lethal defects in rotation and septation of the outflow tract. We previously showed that Fibroblast Growth Factor 8 (FGF8) directs a signaling cascade in the second heart field that regulates critical aspects of OFT morphogenesis. Here we show that in addition to the survival and proliferation cues previously described, FGF8 provides instructive and patterning information to OFT myocardial cells and their progenitors that prevents their aberrant differentiation along a working myocardial program.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling.Development. 2006 Jun;133(12):2419-33. doi: 10.1242/dev.02367. Development. 2006. PMID: 16720879 Free PMC article.
-
Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries.Circ Res. 2010 Feb 19;106(3):495-503. doi: 10.1161/CIRCRESAHA.109.201665. Epub 2009 Dec 24. Circ Res. 2010. PMID: 20035084 Free PMC article.
-
Fgf8 is required for anterior heart field development.Development. 2006 Jun;133(12):2435-45. doi: 10.1242/dev.02408. Development. 2006. PMID: 16720880
-
New developments in the second heart field.Differentiation. 2012 Jul;84(1):17-24. doi: 10.1016/j.diff.2012.03.003. Epub 2012 Apr 21. Differentiation. 2012. PMID: 22521611 Review.
-
Notch and cardiac outflow tract development.Ann N Y Acad Sci. 2010 Feb;1188:184-90. doi: 10.1111/j.1749-6632.2009.05099.x. Ann N Y Acad Sci. 2010. PMID: 20201902 Free PMC article. Review.
Cited by
-
In-Depth Genomic Analysis: The New Challenge in Congenital Heart Disease.Int J Mol Sci. 2024 Feb 1;25(3):1734. doi: 10.3390/ijms25031734. Int J Mol Sci. 2024. PMID: 38339013 Free PMC article. Review.
-
Gata6 functions in zebrafish endoderm to regulate late differentiating arterial pole cardiogenesis.Development. 2024 Sep 1;151(17):dev202895. doi: 10.1242/dev.202895. Epub 2024 Sep 2. Development. 2024. PMID: 39133135 Free PMC article.
References
-
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME, 2006. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res 98, 421–428. - PubMed
-
- Bajolle F, Zaffran S, Meilhac SM, Dandonneau M, Chang T, Kelly RG, Buckingham ME, 2008. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev Biol 313, 25–34. - PubMed
-
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC, 2001. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 103, 2745–2752. - PubMed
-
- Biben C, Hatzistavrou T, Harvey RP, 1998. Expression of NK-2 class homeobox gene Nkx2–6 in foregut endoderm and heart. Mech Dev 73, 125–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

