An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH
- PMID: 37061196
- PMCID: PMC12164378
- DOI: 10.1016/j.jhep.2023.03.038
An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH
Abstract
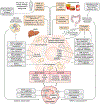
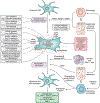
Successful development of treatments for non-alcoholic fatty liver disease and its progressive form, non-alcoholic steatohepatitis (NASH), has been challenging. Because NASH and fibrosis lead to progression towards cirrhosis and clinical outcomes, approaches have either sought to attenuate metabolic dysregulation and cell injury, or directly target the inflammation and fibrosis that ensue. Targets for reducing the activation of inflammatory cascades include nuclear receptor agonists (e.g. resmetirom, lanifibranor, obeticholic acid), modulators of lipotoxicity (e.g. aramchol, acetyl-CoA carboxylase inhibitors) or modification of genetic variants (e.g. PNPLA3 gene silencing). Extrahepatic inflammatory signals from the circulation, adipose tissue or gut are targets of hormonal agonists (semaglutide, tirzepatide, FGF19/FGF21 analogues), microbiota or lifestyle interventions. Stress signals and hepatocyte death activate immune responses, engaging innate (macrophages, innate lymphocyte populations) and adaptive (auto-aggressive T cells) mechanisms. Therapies have also been developed to blunt immune cell activation, recruitment (chemokine receptor inhibitors), and responses (e.g. galectin-3 inhibitors, anti-platelet drugs). The disease-driving pathways of NASH converge to elicit fibrosis, which is reversible. The activation of hepatic stellate cells into matrix-producing myofibroblasts can be inhibited by antagonising soluble factors (e.g. integrins, cytokines), cellular crosstalk (e.g. with macrophages), and agonising nuclear receptor signalling. In advanced fibrosis, cell therapy with restorative macrophages or reprogrammed (CAR) T cells may accelerate repair through hepatic stellate cell deactivation or killing, or by enhancing matrix degradation. Heterogeneity of disease - either due to genetics or divergent disease drivers - is an obstacle to defining effective drugs for all patients with NASH that will be overcome incrementally.
Keywords: FGF-19; FGF-21; FXR; GLP-1; HSC; ILC; NAFLD; OCA; PPAR; TGFβ; THR-β; TR-β; gut-liver axis; scRNA-seq; thyromimetics.
Copyright © 2023 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
FT’s lab has received research funding from Allergan, Bristol-Myers Squibb, Gilead and Inventiva. FT has received honoraria for consulting or lectures from Astra Zeneca, Gilead, AbbVie, BMS, Boehringer, Madrigal, Intercept, Falk, Ionis, Inventiva, Merz, Pfizer, Alnylam, NGM, CSL Behring, Novo Nordisk, Novartis. TP declares no conflict of interest. RL serves as a consultant to Aardvark Therapeutics, Altimmune, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Eli Lilly, Gilead, Glympse bio, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Takeda, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institution received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc. SLF Disclosures: Consulting: 89 Bio, Amgen, Axcella Health, Blade Therapeutics, Bristol Myers Squibb, Can-Fite Biopharma, ChemomAb, Escient Pharmaceuticals, Forbion, Foresite laboratories, Galmed, Gordian Biotechnology, Glycotest, Glympse Bio, Hepgene, In sitro, Morphic Therapeutics, North Sea Therapeutics, Novartis, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Sagimet, Scholar Rock, Surrozen. Stock options: Blade Therapeutics, Escient, Galectin Galmed, Genfit, Glympse, Hepgene, Lifemax, Metacrine, Morphic Therapeutics, Nimbus, North Sea, Therapeutics, Scholar Rock. Research Activities with Commercial Entities): Morphic Therapeutics Novo Nordisk Abalone Bio (SBIR Grant), Galmed.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
At the dawn of potent therapeutics for fatty liver disease - introducing the miniseries on promising pharmacological targets for NASH.J Hepatol. 2023 Aug;79(2):261-262. doi: 10.1016/j.jhep.2023.04.003. J Hepatol. 2023. PMID: 37455046 No abstract available.
References
-
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212–2224. - PubMed
-
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184:2537–2564. - PubMed
-
- Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK130190/DK/NIDDK NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- U01 AA029019/AA/NIAAA NIH HHS/United States
- R01 DK128289/DK/NIDDK NIH HHS/United States
- R01 DK124318/DK/NIDDK NIH HHS/United States
- UL1 TR004419/TR/NCATS NIH HHS/United States
- R01 DK121154/DK/NIDDK NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- P01 HL147835/HL/NHLBI NIH HHS/United States
- R01 DK121378/DK/NIDDK NIH HHS/United States
- P30 CA196521/CA/NCI NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

