Cardiometabolic health, menopausal estrogen therapy and the brain: How effects of estrogens diverge in healthy and unhealthy preclinical models of aging
- PMID: 37061205
- PMCID: PMC10725785
- DOI: 10.1016/j.yfrne.2023.101068
Cardiometabolic health, menopausal estrogen therapy and the brain: How effects of estrogens diverge in healthy and unhealthy preclinical models of aging
Abstract
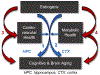
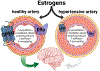
Research in preclinical models indicates that estrogens are neuroprotective and positively impact cognitive aging. However, clinical data are equivocal as to the benefits of menopausal estrogen therapy to the brain and cognition. Pre-existing cardiometabolic disease may modulate mechanisms by which estrogens act, potentially reducing or reversing protections they provide against cognitive decline. In the current review we propose mechanisms by which cardiometabolic disease may alter estrogen effects, including both alterations in actions directly on brain memory systems and actions on cardiometabolic systems, which in turn impact brain memory systems. Consideration of mechanisms by which estrogen administration can exert differential effects dependent upon health phenotype is consistent with the move towards precision or personalized medicine, which aims to determine which treatment interventions will work for which individuals. Understanding effects of estrogens in both healthy and unhealthy models of aging is critical to optimizing the translational link between preclinical and clinical research.
Keywords: Cardiovascular; Cognition; Cortex; Estradiol; Estrogen; Hippocampus; Memory; Menopause; Metabolism.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


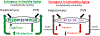
Similar articles
-
Long-term consequences of estrogens administered in midlife on female cognitive aging.Horm Behav. 2015 Aug;74:77-85. doi: 10.1016/j.yhbeh.2015.04.012. Epub 2015 Apr 25. Horm Behav. 2015. PMID: 25917862 Free PMC article. Review.
-
History of Previous Midlife Estradiol Treatment Permanently Alters Interactions of Brain Insulin-like Growth Factor-1 Signaling and Hippocampal Estrogen Synthesis to Enhance Cognitive Aging in a Rat Model of Menopause.J Neurosci. 2022 Oct 19;42(42):7969-7983. doi: 10.1523/JNEUROSCI.0588-22.2022. Epub 2022 Sep 6. J Neurosci. 2022. PMID: 36261268 Free PMC article.
-
Reprint of: From the 90׳s to now: A brief historical perspective on more than two decades of estrogen neuroprotection.Brain Res. 2016 Aug 15;1645:79-82. doi: 10.1016/j.brainres.2016.06.016. Epub 2016 Jun 16. Brain Res. 2016. PMID: 27317847 Free PMC article. Review.
-
From the 90's to now: A brief historical perspective on more than two decades of estrogen neuroprotection.Brain Res. 2016 Feb 15;1633:96-100. doi: 10.1016/j.brainres.2015.12.044. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740397 Free PMC article.
-
The Benefits of Estradiol on Cognitive Aging in Rats Are Independent From Its Effects on Cardiometabolic Health.Endocrinology. 2025 May 19;166(7):bqaf097. doi: 10.1210/endocr/bqaf097. Endocrinology. 2025. PMID: 40420824 Free PMC article.
Cited by
-
Therapeutic Advantages of Isoflavone Glycoside and Aglycone Forms of Sophoricoside in the Amelioration of Postmenopausal Symptoms: Bone Health, Metabolic Regulation, and Systemic Inflammation.Molecules. 2025 May 20;30(10):2218. doi: 10.3390/molecules30102218. Molecules. 2025. PMID: 40430390 Free PMC article.
-
A consideration of the potential benefits and harms of menopausal hormone treatment.PLoS Med. 2025 Apr 10;22(4):e1004567. doi: 10.1371/journal.pmed.1004567. eCollection 2025 Apr. PLoS Med. 2025. PMID: 40208881 Free PMC article.
-
Sex-Specific Vulnerabilities to Subclinical Vascular Brain Injury in Early Late-Life: The Framingham Heart Study.Ann Neurol. 2025 Mar;97(3):460-469. doi: 10.1002/ana.27135. Epub 2024 Nov 14. Ann Neurol. 2025. PMID: 39540324
-
Midlife estradiol treatment reduces the firing rate of liver-related PVN neurons in ovariectomized high-fat diet-fed mice.Am J Physiol Regul Integr Comp Physiol. 2025 Aug 1;329(2):R245-R252. doi: 10.1152/ajpregu.00117.2025. Epub 2025 Jun 26. Am J Physiol Regul Integr Comp Physiol. 2025. PMID: 40568886 Free PMC article.
-
Current Insights in Prolactin Signaling and Ovulatory Function.Int J Mol Sci. 2024 Feb 6;25(4):1976. doi: 10.3390/ijms25041976. Int J Mol Sci. 2024. PMID: 38396659 Free PMC article. Review.
References
-
- Alexandersen P, Tanko LB, Bagger YZ, Qin G, Christiansen C, 2006. The long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric 9, 108–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

