Digital adherence technologies to improve tuberculosis treatment outcomes in China: a cluster-randomised superiority trial
- PMID: 37061308
- PMCID: PMC10126227
- DOI: 10.1016/S2214-109X(23)00068-2
Digital adherence technologies to improve tuberculosis treatment outcomes in China: a cluster-randomised superiority trial
Abstract
Background: Drug-sensitive tuberculosis treatment requires 6 months of therapy, so adherence problems are common. Digital adherence technologies might improve tuberculosis treatment outcomes. We aimed to evaluate the effect of a daily reminder medication monitor, monthly review of adherence data by the health-care provider, and differentiated care for patients with adherence issues, on tuberculosis treatment adherence and outcomes.
Methods: We did a cluster-randomised superiority trial across four prefectures in China. 24 counties or districts (clusters) were randomly assigned (1:1) to intervention or control groups. We enrolled patients aged 18 years or older with GeneXpert-positive, rifampicin-sensitive pulmonary tuberculosis, who were receiving daily fixed-dose combination treatment. Patients in the intervention group received a medication monitor for daily drug-dosing reminders, monthly review of adherence data by health-care provider, and management of poor adherence; and patients in the control group received routine care (silent-mode monitor-measured adherence). Only the independent endpoints review committee who assessed endpoint data for some participants were masked to study group assignment. Patients were followed up (with sputum solid culture) at 12 and 18 months. The primary outcome was a composite of death, loss to follow-up, treatment failure, switch to multidrug-resistant tuberculosis treatment, or tuberculosis recurrence by 18 months from treatment start, analysed in the intention-to-treat population. Analysis accounted for study design with multiple imputation for the primary outcome. This trial is now complete and is registered with ISRCTN, 35812455.
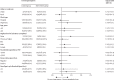
Findings: Between Jan 26, 2017, and April 3, 2019, 15 257 patients were assessed for eligibility and 3074 were enrolled, 2686 (87%) of whom were included in the intention-to-treat population. 1909 (71%) of 2686 patients were male, 777 (29%) were female, and the median age was 44 years (IQR 29-58). By 18 months from treatment start, using multiple imputation for missing outcomes, 239 (16% [geometric mean of cluster-level proportion]) of 1388 patients in the control group and 224 (16%) of 1298 in the intervention group had a primary composite outcome event (289 [62%] of 463 events were loss to follow-up during treatment and 42 [9%] were tuberculosis recurrence). The intervention had no effect on risk of the primary composite outcome (adjusted risk ratio 1·01, 95% CI 0·73-1·40).
Interpretation: Our digital medication monitor intervention had no effect on unfavourable outcomes, which included loss to follow-up during treatment, tuberculosis recurrence, death, and treatment failure. There was a failure to change patient management following identification of treatment non-adherence at monthly reviews. A better understanding of adherence patterns and how they relate to poor outcomes, coupled with a more timely review of adherence data and improved implementation of differentiated care, may be required.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Addressing the adherence challenge in tuberculosis treatment: more than digital technologies.Lancet Glob Health. 2023 May;11(5):e634-e635. doi: 10.1016/S2214-109X(23)00160-2. Lancet Glob Health. 2023. PMID: 37061297 No abstract available.
-
Digital adherence technologies in tuberculosis.Lancet Glob Health. 2023 Sep;11(9):e1341-e1342. doi: 10.1016/S2214-109X(23)00308-X. Lancet Glob Health. 2023. PMID: 37591581 No abstract available.
References
-
- WHO . World Health Organization; Geneva: 2022. Global tuberculosis report 2022.
-
- Chimeh RA, Gafar F, Pradipta IS, et al. Clinical and economic impact of medication non-adherence in drug-susceptible tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2020;24:811–819. - PubMed
-
- Hou WL, Song FJ, Zhang NX, et al. Implementation and community involvement in DOTS strategy: a systematic review of studies in China. Int J Tuberc Lung Dis. 2012;16:1433–1440. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous