Glucose-Induced Activation of mTORC1 is Associated with Hexokinase2 Binding to Sestrins in HEK293T Cells
- PMID: 37061344
- PMCID: PMC10273196
- DOI: 10.1016/j.tjnut.2022.11.021
Glucose-Induced Activation of mTORC1 is Associated with Hexokinase2 Binding to Sestrins in HEK293T Cells
Abstract
Background: Sestrins (SESN1-3) act as proximal sensors in leucine-induced activation of the protein kinase mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1), a key regulator of cell growth and metabolism.
Objective: In the present study, the hypothesis that SESNs also mediate glucose-induced activation of mTORC1 was tested.
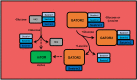
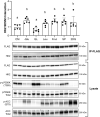
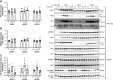
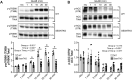
Methods: Rats underwent overnight fasting, and in the morning, either saline or a glucose solution (4 g⋅kg-1 BW/10 mL⋅kg-1) was administered by oral gavage; mTORC1 activation in the tibialis anterior muscle was assessed. To further assess the mechanism through which glucose promotes mTORC1 activation, wild-type (WT) HEK293T and HEK293T cells lacking either all 3 SESNs (SESNTKO) or hexokinase 2 (HK2KO) were deprived of glucose, followed by glucose addback, and mTORC1 activation was assessed. In addition, glucose-induced changes in the association of the SESNs with components of the GAP activity toward the Rags (GATOR2) complex and with hexokinase 2 (HK2) were assessed by co-immunoprecipitation. One- and two-way ANOVA with Tukey post hoc comparisons were used.
Results: Glucose administration to fasted rats promoted mTORC1 activation. Similarly, glucose readdition (GluAB) to the medium of glucose-deprived WT cells also promoted mTORC1 activation. By contrast, SESNTKO cells demonstrated attenuated mTORC1 activation following GluAB compared with WT cells. Interestingly, HK2 associated with all 3 SESNs in a glucose-dependent manner, i.e., HK2 abundance in SESN immunoprecipitates was high in cells deprived of glucose and decreased in response to GluAB. Moreover, similar to SESNTKO cells, the sensitivity of mTORC1 to GluAB was attenuated in HK2KO cells compared with WT cells.
Conclusions: The results of this study demonstrate that the SESNs and HK2 play important roles in glucose-induced mTORC1 activation in HEK293T cells. However, unlike leucine-induced mTORC1 activation, the effect was independent of the changes in SESN-GATOR2 interaction, and instead, it was associated with alterations in the association of SESNs with HK2.
Keywords: glucose; mTORC1; metabolism; nutrient signaling; skeletal muscle.
Copyright © 2022 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
New Insight into the Understanding of Muscle Glycolysis: Sestrins, Key Pivotal Proteins Integrating Glucose and Leucine to Control mTOR Activation.J Nutr. 2023 Apr;153(4):915-916. doi: 10.1016/j.tjnut.2023.01.029. Epub 2023 Feb 3. J Nutr. 2023. PMID: 36796434 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

