A phase II study of neoadjuvant atezolizumab and nab-paclitaxel in patients with anthracycline-resistant early-stage triple-negative breast cancer
- PMID: 37061619
- PMCID: PMC12247189
- DOI: 10.1007/s10549-023-06929-9
A phase II study of neoadjuvant atezolizumab and nab-paclitaxel in patients with anthracycline-resistant early-stage triple-negative breast cancer
Abstract
Purpose: Neoadjuvant anti-PD-(L)1 therapy improves the pathological complete response (pCR) rate in unselected triple-negative breast cancer (TNBC). Given the potential for long-term morbidity from immune-related adverse events (irAEs), optimizing the risk-benefit ratio for these agents in the curative neoadjuvant setting is important. Suboptimal clinical response to initial neoadjuvant therapy (NAT) is associated with low rates of pCR (2-5%) and may define a patient selection strategy for neoadjuvant immune checkpoint blockade. We conducted a single-arm phase II study of atezolizumab and nab-paclitaxel as the second phase of NAT in patients with doxorubicin and cyclophosphamide (AC)-resistant TNBC (NCT02530489).
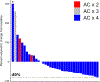
Methods: Patients with stage I-III, AC-resistant TNBC, defined as disease progression or a < 80% reduction in tumor volume after 4 cycles of AC, were eligible. Patients received atezolizumab (1200 mg IV, Q3weeks × 4) and nab-paclitaxel (100 mg/m2 IV,Q1 week × 12) as the second phase of NAT before undergoing surgery followed by adjuvant atezolizumab (1200 mg IV, Q3 weeks, × 4). A two-stage Gehan-type design was employed to detect an improvement in pCR/residual cancer burden class I (RCB-I) rate from 5 to 20%.
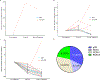
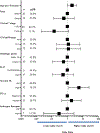
Results: From 2/15/2016 through 1/29/2021, 37 patients with AC-resistant TNBC were enrolled. The pCR/RCB-I rate was 46%. No new safety signals were observed. Seven patients (19%) discontinued atezolizumab due to irAEs.
Conclusion: This study met its primary endpoint, demonstrating a promising signal of activity in this high-risk population (pCR/RCB-I = 46% vs 5% in historical controls), suggesting that a response-adapted approach to the utilization of neoadjuvant immunotherapy should be considered for further evaluation in a randomized clinical trial.
Keywords: Atezolizumab; Chemotherapy resistance; Phase II trial; Triple-negative breast cancer.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflict of Interest
C.Y. has received research funding (to the institution) from Genentech, Gilead, BostonGene, Sanofi, Amgen, Pfizer, Astellas, Novartis and has served on advisory boards for Gilead. E.A.M. has received research support from Roche/Genentech (via the SU2C grant), and Gilead, has served on steering committees for Bristol Myers Squib, Lilly, Roche/Genentech, and has served on scientific advisory boards for AstraZeneca, Exact Sciences (formerly Genomic Health), Merck, Roche/Genentech. R.K.M. has received honoraria from Puma biotechnology, Seattle genetics, Genetech, Novartis, and Astrazeneca; has served in a consulting or advisory role for Sanofi, Novartis, AstraZeneca, Pfizer, Genentech/Roche, Seattle Genetics, Puma Biotechnology; has received research funding (to the institution) from Seattle Genetics, Genetch/Roche, Pfizer, Daiichi Sankyo, AstraZeneca, EMD Serono; has received travel, accommodations, expenses from Genentech, Seattle Genetics. E.A.R. is currently employed at Eli Lilly (previously employed by MD Anderson at the time the study was conducted). W.F.S. is a co-inventor/patent of US Patent No. 11,459,617 “Targeted measure of transcriptional activity related to hormone receptors” issued on 10/4/2022 (applicant proprietor: University of Texas MD Anderson Cancer Center. Licensed to Delphi Diagnostics, Inc. and has co-founder equity from Delphi Diagnostics, Inc. A.T. is related by marriage to an employee of Eli Lilly. D.T. has received research support (to the institution) from Novartis, Pfizer, Polyphor and has served as a consultant to AstraZeneca, Glaxosmithkline, Gilead, Oncopep, Pfizer, Novartis, AMBRX, Personalis, Sermonix, Stemline-Menarini, Puma Biotechnology. S.L.M. is currently employed by Eli Lilly (previously employed by MD Anderson at the time the study was conducted). J.K.L. has received grant or research support from Novartis, Medivation/Pfizer, Genentech, GSK, EMD-Serono, Astra-Zeneca, Medimmune, Zenith, Jounce; participated in Speaker’s Bureau for MedLearning, Physician’s Education Resource, Prime Oncology, Medscape, Clinical Care Options; received honoraria from UpToDate; served on advisory committees or review panels for Astra-Zeneca, Ayala, Pfizer (all uncompensated), NCCN, ASCO, NIH, PDQ, SITC Breast Committee, SWOG Breast Committee. All other authors declare that they have no relevant conflicts of interest.
Figures
References
-
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Investigators IMT (2018) Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379 (22):2108–2121. doi: 10.1056/NEJMoa1809615 - DOI - PubMed
-
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P, Investigators K- (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396 (10265):1817–1828. doi: 10.1016/S0140-6736(20)32531-9 - DOI - PubMed
-
- Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA, Investigators IM (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21 (1):44–59. doi: 10.1016/S1470-2045(19)30689-8 - DOI - PubMed
-
- Emens LA, Adams S, Barrios CH, Dieras V, Iwata H, Loi S, Rugo HS, Schneeweiss A, Winer EP, Patel S, Henschel V, Swat A, Kaul M, Molinero L, Patel S, Chui SY, Schmid P (2021) First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol 32 (8):983–993. doi: 10.1016/j.annonc.2021.05.355 - DOI - PubMed
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O'Shaughnessy J, Investigators K- (2020) Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 382 (9):810–821. doi: 10.1056/NEJMoa1910549 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

