Nirmatrelvir/ritonavir treatment in SARS-CoV-2 positive kidney transplant recipients - a case series with four patients
- PMID: 37061677
- PMCID: PMC10105635
- DOI: 10.1186/s12882-023-03154-w
Nirmatrelvir/ritonavir treatment in SARS-CoV-2 positive kidney transplant recipients - a case series with four patients
Abstract
Background: Despite vaccination coronavirus disease 2019 (COVID-19)-associated mortality caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains high in kidney transplant recipients. Nirmatrelvir is a protease inhibitor with activity against SARS-CoV-2. Nirmatrelvir reduces the risk for mortality and hospitalization, which is approved for treating adults at risk for severe disease. Nirmatrelvir is metabolized by the cytochrome P-450 (CYP) 3A4 isozyme CYP3A4 and is therefore co-administered with the irreversible CYP3A4 inhibitor ritonavir, which results in a drug interaction with tacrolimus. A limited number of patients with nirmatrelvir/ritonavir and tacrolimus therapy after kidney transplantation have been reported to date. It has been reported that tacrolimus was paused during the five-day nirmatrelvir/ritonavir therapy and subtherapeutic tacrolimus levels were observed after finishing nirmatrelvir/ritonavir in two patients. Therefore, optimization of tacrolimus dosing is urgently needed in transplant recipients receiving nirmatrelvir/ritonavir treatment.
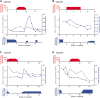
Case presentation: Here, we present our first-hand experience with four patients receiving tacrolimus therapy following kidney transplantation and nirmatrelvir/ritonavir therapy due to COVID-19. Tacrolimus was paused during nirmatrelvir/ritonavir therapy in all patients, which resulted in stable therapeutic tacrolimus levels. Tacrolimus was continued directly after finishing nirmatrelvir/ritonavir to avoid subtherapeutic levels in the first patient treated. This patient received his usual tacrolimus maintenance dose, which resulted in toxic levels. Based on this observation, tacrolimus therapy was continued 24 h after finishing nirmatrelvir/ritonavir treatment at a reduced dose in the subsequent patients. In these patients, therapeutic to supratherapeutic tacrolimus levels were observed despite the therapeutic break and dose reduction.
Discussion and conclusions: Based on altered CYP3A4 metabolism, tacrolimus levels have to be closely monitored after treatment with nirmatrelvir/ritonavir. Our study suggests that tacrolimus treatment should be paused during nirmatrelvir/ritonavir medication and be continued 24 h after completing nirmatrelvir/ritonavir therapy at a reduced dose and under close monitoring. Based on the limited number of patients in this study, results must be interpreted with caution.
Keywords: COVID-19; CYP3A4 interaction; Case series; Kidney transplantation; Nirmatrelvir/ritonavir.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Callaghan CJ, Mumford L, Curtis RMK, Williams SV, Whitaker H, Andrews N, et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients. Transplantation. 2022;106(3):436–46. doi: 10.1097/TP.0000000000004059. - DOI - PMC - PubMed
-
- Udomkarnjananun S, Kerr SJ, Townamchai N, Susantitaphong P, Tulvatana W, Praditpornsilpa K, et al. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep. 2021;11(1):20073. doi: 10.1038/s41598-021-99713-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous