Compensatory remodeling of a septo-hippocampal GABAergic network in the triple transgenic Alzheimer's mouse model
- PMID: 37061718
- PMCID: PMC10105965
- DOI: 10.1186/s12967-023-04078-7
Compensatory remodeling of a septo-hippocampal GABAergic network in the triple transgenic Alzheimer's mouse model
Abstract
Background: Alzheimer's disease (AD) is characterized by a progressive loss of memory that cannot be efficiently managed by currently available AD therapeutics. So far, most treatments for AD that have the potential to improve memory target neural circuits to protect their integrity. However, the vulnerable neural circuits and their dynamic remodeling during AD progression remain largely undefined.
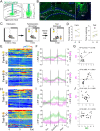
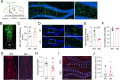
Methods: Circuit-based approaches, including anterograde and retrograde tracing, slice electrophysiology, and fiber photometry, were used to investigate the dynamic structural and functional remodeling of a GABAergic circuit projected from the medial septum (MS) to the dentate gyrus (DG) in 3xTg-AD mice during AD progression.
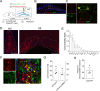
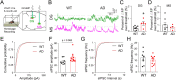
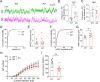
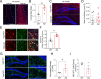
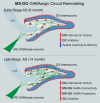
Results: We identified a long-distance GABAergic circuit that couples highly connected MS and DG GABAergic neurons during spatial memory encoding. Furthermore, we found hyperactivity of DG interneurons during early AD, which persisted into late AD stages. Interestingly, MS GABAergic projections developed a series of adaptive strategies to combat DG interneuron hyperactivity. During early-stage AD, MS-DG GABAergic projections exhibit increased inhibitory synaptic strength onto DG interneurons to inhibit their activities. During late-stage AD, MS-DG GABAergic projections form higher anatomical connectivity with DG interneurons and exhibit aberrant outgrowth to increase the inhibition onto DG interneurons.
Conclusion: We report the structural and functional remodeling of the MS-DG GABAergic circuit during disease progression in 3xTg-AD mice. Dynamic MS-DG GABAergic circuit remodeling represents a compensatory mechanism to combat DG interneuron hyperactivity induced by reduced GABA transmission.
Keywords: Alzheimer’s disease; Dentate gyrus; Medial septum; Septo-hippocampal GABAergic network.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Zhu CW, Livote EE, Scarmeas N, Albert M, Brandt J, Blacker D, Sano M, Stern Y. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement. 2013;9:733–40. doi: 10.1016/j.jalz.2012.09.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH111773/MH/NIMH NIH HHS/United States
- U54 HD079124/HD/NICHD NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- RF1 AG071000/AG/NIA NIH HHS/United States
- R01 NS121300/NS/NINDS NIH HHS/United States
- MH111773-01/NH/NIH HHS/United States
- U54 CA156733/CA/NCI NIH HHS/United States
- R01 MH122692/MH/NIMH NIH HHS/United States
- NS104530/NH/NIH HHS/United States
- RF1AG071000-01/NH/NIH HHS/United States
- MH122692-01/NH/NIH HHS/United States
- F31 AG066504/AG/NIA NIH HHS/United States
- R01 MH132222/MH/NIMH NIH HHS/United States
- AG058160/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

