Incorporation, fate, and turnover of free fatty acids in cyanobacteria
- PMID: 37061785
- PMCID: PMC10114076
- DOI: 10.1093/femsre/fuad015
Incorporation, fate, and turnover of free fatty acids in cyanobacteria
Abstract
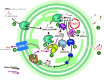
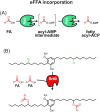
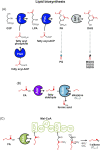
Fatty acids are important molecules in bioenergetics and also in industry. The phylum cyanobacteria consists of a group of prokaryotes that typically carry out oxygenic photosynthesis with water as an electron donor and use carbon dioxide as a carbon source to generate a range of biomolecules, including fatty acids. They are also able to import exogenous free fatty acids and direct them to biosynthetic pathways. Here, we review current knowledge on mechanisms and regulation of free fatty acid transport into cyanobacterial cells, their subsequent activation and use in the synthesis of fatty acid-containing biomolecules such as glycolipids and alka(e)nes, as well as recycling of free fatty acids derived from such molecules. This review also covers efforts in the engineering of such cyanobacterial fatty acid-associated pathways en route to optimized biofuel production.
Keywords: acyl–acyl carrier protein; acyl–acyl carrier protein synthetase; biofuel; cyanobacteria; exogenous free fatty acid; lipid.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures



Similar articles
-
Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling.Plant Physiol. 2010 Mar;152(3):1598-610. doi: 10.1104/pp.109.148007. Epub 2010 Jan 8. Plant Physiol. 2010. PMID: 20061450 Free PMC article.
-
Fatty aldehydes in cyanobacteria are a metabolically flexible precursor for a diversity of biofuel products.PLoS One. 2013;8(3):e58307. doi: 10.1371/journal.pone.0058307. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23505484 Free PMC article.
-
High Light Induced Alka(e)ne Biodegradation for Lipid and Redox Homeostasis in Cyanobacteria.Front Microbiol. 2020 Jul 17;11:1659. doi: 10.3389/fmicb.2020.01659. eCollection 2020. Front Microbiol. 2020. PMID: 32765469 Free PMC article.
-
Photosynthetic Conversion of Carbon Dioxide to Oleochemicals by Cyanobacteria: Recent Advances and Future Perspectives.Front Microbiol. 2020 Apr 17;11:634. doi: 10.3389/fmicb.2020.00634. eCollection 2020. Front Microbiol. 2020. PMID: 32362881 Free PMC article. Review.
-
Distribution and dynamics of electron transport complexes in cyanobacterial thylakoid membranes.Biochim Biophys Acta. 2016 Mar;1857(3):256-65. doi: 10.1016/j.bbabio.2015.11.010. Epub 2015 Nov 24. Biochim Biophys Acta. 2016. PMID: 26619924 Free PMC article. Review.
Cited by
-
EXCRETE workflow enables deep proteomics of the microbial extracellular environment.Commun Biol. 2024 Sep 25;7(1):1189. doi: 10.1038/s42003-024-06910-2. Commun Biol. 2024. PMID: 39322645 Free PMC article.
-
Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria.Metabolites. 2024 Jul 6;14(7):378. doi: 10.3390/metabo14070378. Metabolites. 2024. PMID: 39057701 Free PMC article. Review.
-
Removal of cyanobacterial harmful algal blooms (HABs) from contaminated local park lake using Ganoderma lucidum mycelial pellets.Heliyon. 2024 Dec 15;11(1):e41205. doi: 10.1016/j.heliyon.2024.e41205. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39811270 Free PMC article.
-
Uncovering the substrate of olefin synthase loading domains in cyanobacteria Picosynechococcus sp. strain PCC 7002.RSC Chem Biol. 2025 Jan 14;6(2):307-316. doi: 10.1039/d4cb00234b. eCollection 2025 Feb 5. RSC Chem Biol. 2025. PMID: 39817101 Free PMC article.
-
Application of Cyanobacteria as Chassis Cells in Synthetic Biology.Microorganisms. 2024 Jul 5;12(7):1375. doi: 10.3390/microorganisms12071375. Microorganisms. 2024. PMID: 39065143 Free PMC article. Review.
References
-
- Afonso TB, Costa MS, Rezende De Castro Ret al. . Bartolosides E-K from a marine coccoid cyanobacterium. J Nat Prod. 2016;79:2504–13. - PubMed
-
- Arai M, Hayashi Y, Kudo H. Cyanobacterial enzymes for bioalkane production. In:Zhang W, Song X (ed.), Synthetic Biology of Cyanobacteria. Advances in Experimental Medicine and Biology, Vol.1080. Singapore: Springer, 2018. - PubMed
-
- Arias-Barrau E, Dirusso CC, Black PN. Methods to monitor fatty acid transport proceeding through vectorial acylation. In: Armstrong D (ed.), Lipidomics. Methods in Molecuar Biology™,Vol. 580, Humana Press, 2009, 233–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

