EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS)
- PMID: 37061848
- PMCID: PMC10105878
- DOI: 10.1093/europace/euad043
EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS)
Abstract
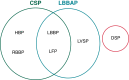
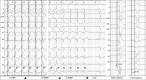
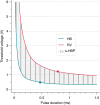
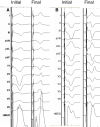
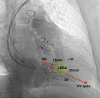
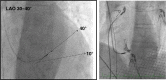
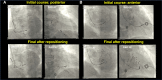
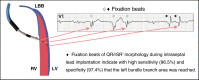
Conduction system pacing (CSP) has emerged as a more physiological alternative to right ventricular pacing and is also being used in selected cases for cardiac resynchronization therapy. His bundle pacing was first introduced over two decades ago and its use has risen over the last five years with the advent of tools which have facilitated implantation. Left bundle branch area pacing is more recent but its adoption is growing fast due to a wider target area and excellent electrical parameters. Nevertheless, as with any intervention, proper technique is a prerequisite for safe and effective delivery of therapy. This document aims to standardize the procedure and to provide a framework for physicians who wish to start CSP implantation, or who wish to improve their technique.
Keywords: Conduction system pacing; Device implantation; His bundle pacing; Left bundle branch area pacing.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures












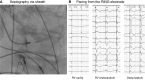
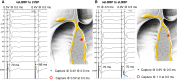


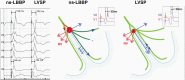



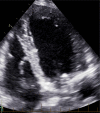

References
-
- Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom-Lundqvist Cet al. . 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. - PubMed
-
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. . 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. - PubMed
-
- Burri H, Starck C. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS)-a role for postoperative ultrasound? Authors’ reply. Europace 2021;24:523–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

