Tumor-Agnostic Precision Medicine from the AACR GENIE Database: Clinical Implications
- PMID: 37061987
- PMCID: PMC10390861
- DOI: 10.1158/1078-0432.CCR-23-0090
Tumor-Agnostic Precision Medicine from the AACR GENIE Database: Clinical Implications
Abstract
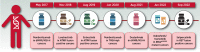
Biomarker-driven cancer therapy has revolutionized precision oncology. With a better understanding of tumor biology, tissue-agnostic targets have been characterized and explored, which ultimately led to therapeutics with pan-cancer efficacy. To date, five molecular biomarkers have obtained FDA tissue-agnostic approval for targeted therapies and immunotherapies. Those include BRAFV600E mutations, RET fusions, NTRK fusions, high tumor mutation burden (TMB), and deficient mismatch repair/high microsatellite instability (dMMR/MSI-High). Herein, we have used data from AACR project GENIE to explore the clinico-genomic landscape of these alterations. AACR GENIE is a publicly accessible registry of genomic data from multiple collaborating cancer centers. Current database (version 13.0) includes sequencing data of 168,423 samples collected from patients with different cancers. We were able to identify BRAFV600E, RET fusions, NTRK fusions, and high TMB in 2.9%, 1.6%, 1.5%, and 15.2% of pan-cancer samples, respectively. In this article, we describe the distribution of those tissue-agnostic targets among different cancer types. In addition, we summarize the current prospect on the biology of these alterations and evidence on approved drugs, including pembrolizumab, dostarilmab, larotrectinib, entrectinib, selpercatinib, and dabrafenib/trametinib combination.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Russell WO. The pathologic diagnosis of cancer–a crescendo of importance in current and future therapies. Ward burdick award lecture. Am J Clin Pathol 1980;73:3–11. - PubMed
-
- Subbiah V, Wolf PJ, Konda B, Spira HKA, Weiss J, Takeda M, et al. . Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol 2022;23:1261–73. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

