Switchable CAR T cell strategy against osteosarcoma
- PMID: 37062034
- PMCID: PMC10361906
- DOI: 10.1007/s00262-023-03437-z
Switchable CAR T cell strategy against osteosarcoma
Abstract
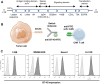
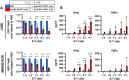
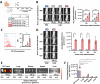
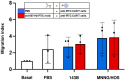
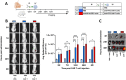
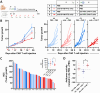
Immunotherapy with chimeric antigen receptor T (CAR T) cells has changed the treatment of hematological malignances, but they are still a challenge for solid tumors, including pediatric sarcomas. Here, we report a switchable CAR T cell strategy based on anti-FITC CAR T cells and a switch molecule conjugated with FITC for targeting osteosarcoma (OS) tumors. As a potential target, we analyzed the expression of B7-H3, an immune checkpoint inhibitor, in OS cell lines. In addition, we evaluate the capacity of an anti-B7-H3 monoclonal antibody conjugated with FITC (anti-B7-H3-FITC mAb) to control the antitumor activity of anti-FITC CAR T cells. The effector functions of anti-FITC CAR T cells against OS, measured in vitro by tumor cell killing activity and cytokine production, are dependent on the presence of the anti-B7-H3-FITC mAb switch. Moreover, OS cells stimulate anti-FITC CAR T cells migration. In vivo, anti-B7-H3 mAb penetrates in the tumor and binds 143B OS tumor cells. Furthermore, anti-FITC CAR T cells reach tumor region and exert antitumor effect in an OS NSG mouse model only in the presence of the switch molecule. We demonstrate that anti-B7-H3-FITC mAb redirects the cytotoxic activity of anti-FITC CAR T cells against OS tumors suggesting that switchable CAR T cell platforms might be a plausible strategy against OS.
Keywords: B7-H3; CAR T; Immunotherapy; Osteosarcoma.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
References
-
- Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

