Web-based tool for cancer family history collection: A prospective randomized controlled trial
- PMID: 37062188
- PMCID: PMC10310435
- DOI: 10.1016/j.ygyno.2023.04.001
Web-based tool for cancer family history collection: A prospective randomized controlled trial
Abstract
Objectives: Approximately 1% of individuals have a hereditary cancer predisposition syndrome, however, the majority are not aware. Collecting a cancer family history (CFH) can triage patients to receive genetic testing. To rigorously assess different methods of CFH collection, we compared a web-based tool (WBT) to usual care (clinician collects CFH) in a randomized controlled trial.
Methods: New gynecologic oncology patients (seen 9/2019-9/2021) were randomized to one of three arms in a 2:2:1 allocation ratio: 1) usual care clinician CFH collection, 2) WBT completed at home, or 3) WBT completed in office. The WBT generated a cancer-focused pedigree and scores on eight validated cancer risk models. The primary outcome was collection of an adequate CFH (based on established guidelines) with usual care versus the WBT.
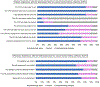
Results: We enrolled 250 participants (usual care - 110; WBT home - 105; WBT office - 35 [closed early due to COVID-19]). Within WBT arms, 109 (78%) participants completed the tool, with higher completion for office versus home (33 [94%] vs. 76 [72%], P = 0.008). Among participants completing the WBT, 63 (58%) had an adequate CFH versus 5 (5%) for usual care (P < 0.001). Participants completing the WBT were significantly more likely to complete genetic counseling (34 [31%] vs. 15 [14%], P = 0.002) and genetic testing (20 [18%] vs. 9 [8%], P = 0.029). Participant and provider WBT experience was favorable.
Conclusions: WBTs for CFH collection are a promising application of health information technology, resulting in more comprehensive CFH and a significantly greater percentage of participants completing genetic counseling and testing.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics.
Figures
References
-
- Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG. 2019. - PubMed
-
- Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–7. - PubMed
-
- Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. 2017;146:217–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous