Replacement of dietary saturated with unsaturated fatty acids is associated with beneficial effects on lipidome metabolites: a secondary analysis of a randomized trial
- PMID: 37062359
- PMCID: PMC10315407
- DOI: 10.1016/j.ajcnut.2023.03.024
Replacement of dietary saturated with unsaturated fatty acids is associated with beneficial effects on lipidome metabolites: a secondary analysis of a randomized trial
Abstract
Background: The effects of replacing dietary saturated fatty acids (SFAs) with monounsaturated fatty acids (MUFAs) and/or polyunsaturated fatty acids (PUFAs) on the plasma lipidome in relation to the cardiometabolic disease (CMD) risk is poorly understood.
Objectives: We aimed to assess the impact of substituting dietary SFAs with unsaturated fatty acids (UFAs) on the plasma lipidome and examine the relationship between lipid metabolites modulated by diet and CMD risk.
Methods: Plasma fatty acid (FA) concentrations among 16 lipid classes (within-class FAs) were measured in a subgroup from the Dietary Intervention and VAScular function (DIVAS) parallel randomized controlled trial (n = 113/195), which consisted of three 16-wk diets enriched in SFAs (target SFA:MUFA:n-6PUFA ratio = 17:11:4% total energy [TE]), MUFAs (9:19:4% TE), or a MUFA/PUFA mixture (9:13:10% TE). Similar lipidomics analyses were conducted in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study (specific case/cohorts: n = 775/1886 for type 2 diabetes [T2D], n = 551/1671 for cardiovascular disease [CVD]). Multiple linear regression and multivariable Cox models identified within-class FAs sensitive to replacement of dietary SFA with UFA in DIVAS and their association with CMD risk in EPIC-Potsdam. Elastic-net regression models identified within-class FAs associated with changes in CMD risk markers post-DIVAS interventions.
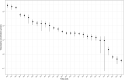
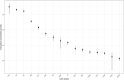
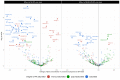
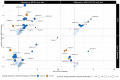
Results: DIVAS high-UFA interventions reduced plasma within-class FAs associated with a higher CVD risk in EPIC-Potsdam, especially SFA-containing glycerolipids and sphingolipids (e.g., diacylglycerol (20:0) z-score = -1.08; SE = 0.17; P value < 10-8), whereas they increased those inversely associated with CVD risk. The results on T2D were less clear. Specific sphingolipids and phospholipids were associated with changes in markers of endothelial function and ambulatory blood pressure, whereas higher low-density lipoprotein cholesterol concentrations were characterized by higher plasma glycerolipids containing lauric and stearic acids.
Conclusions: These results suggest a mediating role of plasma lipid metabolites in the association between dietary fat and CMD risk. Future research combining interventional and observational findings will further our understanding of the role of dietary fat in CMD etiology. This trial was registered in ClinicalTrials.gov as NCT01478958.
Keywords: EPIC-Potsdam; cardiovascular disease; dietary fat; lipidomics; randomized controlled trial; type 2 diabetes.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study.Am J Clin Nutr. 2015 Jul;102(1):40-8. doi: 10.3945/ajcn.114.097089. Epub 2015 May 27. Am J Clin Nutr. 2015. PMID: 26016869 Clinical Trial.
-
Development of a food-exchange model to replace saturated fat with MUFAs and n-6 PUFAs in adults at moderate cardiovascular risk.J Nutr. 2014 Jun;144(6):846-55. doi: 10.3945/jn.114.190645. Epub 2014 Apr 9. J Nutr. 2014. PMID: 24717370 Clinical Trial.
-
Replacement of dietary saturated fat with unsaturated fats increases numbers of circulating endothelial progenitor cells and decreases numbers of microparticles: findings from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study.Am J Clin Nutr. 2018 Jun 1;107(6):876-882. doi: 10.1093/ajcn/nqy018. Am J Clin Nutr. 2018. PMID: 29741564 Clinical Trial.
-
Association of circulating fatty acids with cardiovascular disease risk: analysis of individual-level data in three large prospective cohorts and updated meta-analysis.Eur J Prev Cardiol. 2025 Feb 18;32(3):233-246. doi: 10.1093/eurjpc/zwae315. Eur J Prev Cardiol. 2025. PMID: 39365172 Free PMC article.
-
Emerging nutrition science on fatty acids and cardiovascular disease: nutritionists' perspectives.Adv Nutr. 2015 May 15;6(3):326S-37S. doi: 10.3945/an.114.006981. Print 2015 May. Adv Nutr. 2015. PMID: 25979506 Free PMC article. Review.
Cited by
-
Dietary fats and cardiometabolic health-from public health to personalised nutrition: 'One for all' and 'all for one'.Nutr Bull. 2025 Mar;50(1):132-141. doi: 10.1111/nbu.12722. Epub 2025 Jan 20. Nutr Bull. 2025. PMID: 39833097 Free PMC article.
-
Substitution of dietary monounsaturated fatty acids from olive oil for saturated fatty acids from lard increases low-density lipoprotein apolipoprotein B-100 fractional catabolic rate in subjects with dyslipidemia associated with insulin resistance: a randomized controlled trial.Am J Clin Nutr. 2024 May;119(5):1270-1279. doi: 10.1016/j.ajcnut.2024.03.015. Epub 2024 Mar 20. Am J Clin Nutr. 2024. PMID: 38518848 Free PMC article. Clinical Trial.
-
Metabolite Profiles in Response to Dietary Interventions for Management of Blood Pressure: A Systematic Review.Curr Nutr Rep. 2025 Jun 20;14(1):82. doi: 10.1007/s13668-025-00676-7. Curr Nutr Rep. 2025. PMID: 40540135 Free PMC article. Review.
-
Isoliquiritigenin in combination with visceral adipose tissue and related markers as a predictive tool for nonalcoholic fatty liver disease.J Physiol Biochem. 2024 Aug;80(3):639-653. doi: 10.1007/s13105-023-00998-6. Epub 2023 Nov 24. J Physiol Biochem. 2024. PMID: 37996653 Free PMC article.
-
Heart-healthy diets including phytostanol ester consumption to reduce the risk of atherosclerotic cardiovascular diseases. A clinical review.Lipids Health Dis. 2024 Oct 21;23(1):341. doi: 10.1186/s12944-024-02330-7. Lipids Health Dis. 2024. PMID: 39434087 Free PMC article. Review.
References
-
- Report of an expert consultation [Internet] Rome: Food and Agriculture Organization of the United Nations; 2010. Fats and fatty acids in human nutrition. Report no. 91.
-
- Saturated fats and health . 2019. Scientific Advisory Committee on Nutrition.https://assets.publishing.service.gov.uk/government/uploads/system/uploa... [cited August 1, 2019]; Available from:
-
- William L., Daniel L. 2nd ed. Academic Press; Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Franscisco, Singapore, sydney, Tokyo: 2013. Encyclopedia of Biological Chemistry II.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

