Visualizing reactive astrocyte-neuron interaction in Alzheimer's disease using 11C-acetate and 18F-FDG
- PMID: 37062541
- PMCID: PMC10517195
- DOI: 10.1093/brain/awad037
Visualizing reactive astrocyte-neuron interaction in Alzheimer's disease using 11C-acetate and 18F-FDG
Abstract
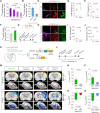
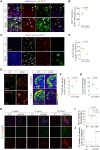
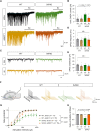
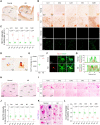
Reactive astrogliosis is a hallmark of Alzheimer's disease (AD). However, a clinically validated neuroimaging probe to visualize the reactive astrogliosis is yet to be discovered. Here, we show that PET imaging with 11C-acetate and 18F-fluorodeoxyglucose (18F-FDG) functionally visualizes the reactive astrocyte-mediated neuronal hypometabolism in the brains with neuroinflammation and AD. To investigate the alterations of acetate and glucose metabolism in the diseased brains and their impact on the AD pathology, we adopted multifaceted approaches including microPET imaging, autoradiography, immunohistochemistry, metabolomics, and electrophysiology. Two AD rodent models, APP/PS1 and 5xFAD transgenic mice, one adenovirus-induced rat model of reactive astrogliosis, and post-mortem human brain tissues were used in this study. We further curated a proof-of-concept human study that included 11C-acetate and 18F-FDG PET imaging analyses along with neuropsychological assessments from 11 AD patients and 10 healthy control subjects. We demonstrate that reactive astrocytes excessively absorb acetate through elevated monocarboxylate transporter-1 (MCT1) in rodent models of both reactive astrogliosis and AD. The elevated acetate uptake is associated with reactive astrogliosis and boosts the aberrant astrocytic GABA synthesis when amyloid-β is present. The excessive astrocytic GABA subsequently suppresses neuronal activity, which could lead to glucose uptake through decreased glucose transporter-3 in the diseased brains. We further demonstrate that 11C-acetate uptake was significantly increased in the entorhinal cortex, hippocampus and temporo-parietal neocortex of the AD patients compared to the healthy controls, while 18F-FDG uptake was significantly reduced in the same regions. Additionally, we discover a strong correlation between the patients' cognitive function and the PET signals of both 11C-acetate and 18F-FDG. We demonstrate the potential value of PET imaging with 11C-acetate and 18F-FDG by visualizing reactive astrogliosis and the associated neuronal glucose hypometablosim for AD patients. Our findings further suggest that the acetate-boosted reactive astrocyte-neuron interaction could contribute to the cognitive decline in AD.
Keywords: 11C-Acetate; 18F-Fluorodeoxyglucose; Alzheimer’s disease; PET imaging; monocarboxylate transporter 1 (MCT1); reactive astrocyte.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures

Comment in
-
Path from discovery to recovery: therapeutic and diagnostic advances in Alzheimer's dementia.J Neurol. 2023 Aug;270(8):4151-4153. doi: 10.1007/s00415-023-11840-w. Epub 2023 Jul 3. J Neurol. 2023. PMID: 37394517 Free PMC article. No abstract available.
References
-
- Chun H, Lee CJ. Reactive astrocytes in Alzheimer’s disease: A double-edged sword. Neurosci Res. 2018;126:44–52. - PubMed
-
- Carter SF, Scholl M, Almkvist O, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: A multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53:37–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

