Systematic review and pooled analysis of randomized controlled trials in countries of the Gulf Cooperation Council (GCC): Methods and quality assessment
- PMID: 37062556
- PMCID: PMC10153608
- DOI: 10.15537/smj.2023.44.4.20220664
Systematic review and pooled analysis of randomized controlled trials in countries of the Gulf Cooperation Council (GCC): Methods and quality assessment
Abstract
Objectives: To describe variations in characteristics of randomized controlled trials conducted in the Gulf Cooperation Council (GCC) countries, and critically appraising the quality of design, conduct and analysis of the trials.
Methods: We carried out a systematically comprehensive electronic search of articles published between 1990 and 2018 and indexed in several databases: i) MEDLINE/PubMed, ii) EMBASE, iii) Cochrane Central Register of Controlled Trials (CENTRAL), iv) ClinicalTrials.gov, and v) World Health Organization International Clinical Trials Registry Platform. We summarized the overall risk of bias present in all analyzed studies using the Cochrane Collaboration risk of bias tool (CCRBT).
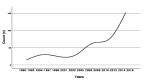
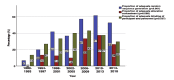
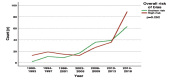
Results: A remarkable shift in numbers of publications from 2006 onwards was found. The largest number of publications were from Saudi Arabia and consisted of hospitals/clinics based studies. Lack of randomization was found in the majority of reports, and nearly three-fourth of the studies involved the use of intention-to-treat (ITT) principle. However, the proportion of adequately generated random sequence methods has increased yearly, and this increase accounted for a relatively large proportion over the latter half of the studied period (p<0.001), in contrast to the proportion of allocation concealment and blinding. Journal impact factor was significantly correlated with the quality of random sequence generation (r=0.145; p=0.014).
Conclusion: The randomization methods have gained more attention over the last 3 decades. Secondly, Journal impact factor can serve as an indicator of randomization quality. To mitigate the large rate of overall high risk of bias in GCC studies, high-quality trials must be considered by ensuring adequate allocation concealment and blinding methods. PROSPERO No. ID: CRD42022310331.
Keywords: Arab world; Clinical Trial; bias; systematic review.
Copyright: © Saudi Medical Journal.
Figures
References
-
- Uetani K, Nakayama T, Ikai H, Yonemoto N, Moher D.. Quality of Reports on Randomized Controlled Trials Conducted in Japan: Evaluation of Adherence to the CONSORT Statement. Intern Med 2009; 48: 307–313. - PubMed
-
- Lim SM, Shin ES, Lee SH, Seo KH, Jung YM, Jang JE.. Tools for assessing quality and risk of bias by levels of evidence. J Korean Med Assoc 2011; 54: 419–429.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

