Targeted Axillary Dissection with 125I Seed Placement Before Neoadjuvant Chemotherapy in a Danish Multicenter Cohort
- PMID: 37062781
- PMCID: PMC10250439
- DOI: 10.1245/s10434-023-13432-4
Targeted Axillary Dissection with 125I Seed Placement Before Neoadjuvant Chemotherapy in a Danish Multicenter Cohort
Abstract
Background: Targeted axillary dissection (TAD), with marking of the metastatic lymph node before neoadjuvant chemotherapy (NACT), is increasingly used for breast cancer axillary staging. In the case of axillary pathological complete response (ax-pCR), axillary lymph node clearance can be omitted. Several marking methods exist, most using re-marking before surgery. Feasibility, learning curve, and identification rate (IR) vary. Marking with 125I seed before NACT makes re-marking at surgery redundant, possibly increasing feasibility and IR. Here, TAD with 125I seed placed before NACT is evaluated in a Danish multicenter cohort.
Methods: Patients staged with 125I TAD in Denmark between 1 January 2016 and 31 August 2021 were included. Patients were identified in radioactivity-emitting implant registries at the radiology departments and from the Danish Breast Cancer Group database. Data were extracted from patients' medical records. Information on patient/tumor characteristics, 125I seed activity, marking period, TAD success, number of sentinel nodes (SNs), the histopathological status of excised nodes, and whether the marked lymph node (MLN) was an SN were registered.
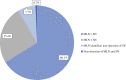
Results: 142 patients were included. The IR of the MLN was 99.3%, and the IR of the SLNB was 91.5%. TAD success was 91.5%. Minor challenges in marking or removal of the MLN were noted in three patients. In 72.3% of the patients, the MLN was a sentinel node. Overall, 40.8% had axillary pCR.
Conclusion: TAD with 125I seed marking before NACT is feasible without re-marking at surgery and with only minor surgical challenges. The IR is high. Staging with TAD spares 41% of breast cancer patients an axillary dissection.
© 2023. The Author(s).
Figures
Comment in
-
ASO Author Reflections: Feasibility of 125I Seed Targeted Axillary Dissection.Ann Surg Oncol. 2023 Jul;30(7):4143-4144. doi: 10.1245/s10434-023-13446-y. Epub 2023 Apr 8. Ann Surg Oncol. 2023. PMID: 37029862 No abstract available.
References
-
- Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative Ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25(3):784–791. doi: 10.1245/s10434-017-6270-z. - DOI - PubMed
-
- Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021 doi: 10.1001/jamasurg.2021.0891. - DOI - PMC - PubMed
-
- El Hage CH, Headon H, El Tokhy O, Heeney J, Kasem A, Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am J Surg. 2016;212(5):969–981. doi: 10.1016/j.amjsurg.2016.07.018. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical