Protoplast isolation and transient transformation system for Ginkgo biloba L
- PMID: 37063206
- PMCID: PMC10099357
- DOI: 10.3389/fpls.2023.1145754
Protoplast isolation and transient transformation system for Ginkgo biloba L
Abstract
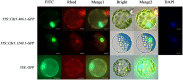
Ginkgo biloba L. has a unique evolutionary status. Owing to its high medicinal and ornamental value, ginkgo has also recently become a research hotspot. However, the large genome and long juvenile period, as well as the lack of an effective genetic transformation system, have hindered gaining a full understanding of the comprehensive functions of ginkgo genes. At present, heterologous expression of genes in model plants is the primary method used in ginkgo-related research; however, these distant plant model relatives limit reliable interpretation of the results for direct applications in ginkgo breeding. To overcome these limitations, in this study, an efficient isolation and transient expression system for ginkgo protoplasts was established. A large number of intact and homogeneous ginkgo mesophyll protoplasts were isolated using 2% cellulase and 0.25% pectinase in 0.4 M mannitol. The activity of these protoplasts remained above 90% even after 24 h. Furthermore, when the concentration of the polyethylene glycol 4000 solution was 30%-40% (w/v), the transformation efficiency of the protoplasts reached 40%. Finally, the reliability of the system was verified using subcellular localization, transient overexpression, and protein interaction experiments with ginkgo genes, thereby providing a technical platform for the identification and analysis of ginkgo gene functions. The proposed method partially compensates for the limitations associated with the lack of a genetic transformation system and provides technical support to expand research on elucidating the functions of ginkgo genes.
Keywords: Ginkgo biloba L.; PEG-mediated transformation; protein interaction; protoplasts; transient expression.
Copyright © 2023 Han, Rong, Feng, Xin, Luan, Zhou, Xu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Isolation, Purification, and Application of Protoplasts and Transient Expression Systems in Plants.Int J Mol Sci. 2023 Nov 29;24(23):16892. doi: 10.3390/ijms242316892. Int J Mol Sci. 2023. PMID: 38069215 Free PMC article. Review.
-
Direct leaf-peeling method for areca protoplasts: a simple and efficient system for protoplast isolation and transformation in areca palm (Areca catechu).BMC Plant Biol. 2023 Jan 26;23(1):56. doi: 10.1186/s12870-023-04048-7. BMC Plant Biol. 2023. PMID: 36698067 Free PMC article.
-
Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz).BMC Biotechnol. 2017 Mar 14;17(1):29. doi: 10.1186/s12896-017-0349-2. BMC Biotechnol. 2017. PMID: 28292294 Free PMC article.
-
Isolation, purification and PEG-mediated transient expression of mesophyll protoplasts in Camellia oleifera.Plant Methods. 2022 Dec 22;18(1):141. doi: 10.1186/s13007-022-00972-1. Plant Methods. 2022. PMID: 36550558 Free PMC article.
-
Isolation, culture, and transient transformation of plant protoplasts.Curr Protoc Cell Biol. 2014 Jun 3;63:2.8.1-17. doi: 10.1002/0471143030.cb0208s63. Curr Protoc Cell Biol. 2014. PMID: 24894837 Review.
Cited by
-
Establishment of efficient hypocotyl-derived protoplast isolation and its application in soybean (Glycine max [L.] Merr.).Front Plant Sci. 2025 May 20;16:1587927. doi: 10.3389/fpls.2025.1587927. eCollection 2025. Front Plant Sci. 2025. PMID: 40464005 Free PMC article.
-
A Comparison of DNA-Methylation during Protoplast Culture of Ponkan Mandarin (Citrus reticulata Blanco) and Tobacco (Nicotiana tabacum L.).Plants (Basel). 2024 Oct 15;13(20):2878. doi: 10.3390/plants13202878. Plants (Basel). 2024. PMID: 39458825 Free PMC article.
-
Optimization of preparation and transformation of protoplasts from Populus simonii × P. nigra leaves and subcellular localization of the major latex protein 328 (MLP328).Plant Methods. 2024 Jan 4;20(1):3. doi: 10.1186/s13007-023-01128-5. Plant Methods. 2024. PMID: 38178205 Free PMC article.
-
Isolation, Purification, and Application of Protoplasts and Transient Expression Systems in Plants.Int J Mol Sci. 2023 Nov 29;24(23):16892. doi: 10.3390/ijms242316892. Int J Mol Sci. 2023. PMID: 38069215 Free PMC article. Review.
-
Development of Protoplast-Based Gene Editing System for Areca Palm.Plants (Basel). 2025 Mar 7;14(6):832. doi: 10.3390/plants14060832. Plants (Basel). 2025. PMID: 40265738 Free PMC article.
References
-
- Bai L., Cheng Y., She J., He Z., Liu H., Zhang G., et al. . (2020). Development of an efficient protoplast isolation and transfection system for castor bean (Ricinus communis l.). Plant Cell Tiss Organ Cult 143, 457–464. doi: 10.1007/s11240-020-01932-0 - DOI
-
- Cao J., Yao D., Lin F., Jiang M. (2014). PEG-mediated transient gene expression and silencing system in maize mesophyll protoplasts: a valuable tool for signal transduction study in maize. Acta Physiol. Plant 36, 1271–1281. doi: 10.1007/s11738-014-1508-x - DOI
-
- Choury Z., Meschini R., Dell’Orso A., Fardusi M. J., Mugnozza G. S., Kuzminsky E. (2018). Optimized conditions for the isolation of mesophyll protoplasts along the growing season from arbutus unedo and their use in single cell gel electrophoresis. Plant Cell Tiss Organ Cult 132, 535–543. doi: 10.1007/s11240-017-1349-6 - DOI
LinkOut - more resources
Full Text Sources

