Astrocytes underlie a faster-onset antidepressant effect of hypidone hydrochloride (YL-0919)
- PMID: 37063256
- PMCID: PMC10090319
- DOI: 10.3389/fphar.2023.1175938
Astrocytes underlie a faster-onset antidepressant effect of hypidone hydrochloride (YL-0919)
Abstract
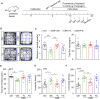
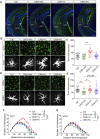
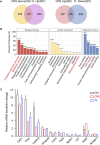
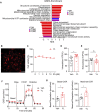
Introduction: Major depression disorder (MDD) is a common and potentially life-threatening mental illness; however, data on its pathogenesis and effective therapeutic measures are lacking. Pathological changes in astrocytes play a pivotal role in MDD. While hypidone hydrochloride (YL-0919), an independently developed antidepressant, has shown rapid action with low side effects, its underlying astrocyte-specific mechanisms remain unclear. Methods: In our study, mice were exposed to chronic restraint stress (CRS) for 14 days or concomitantly administered YL-0919/fluoxetine. Behavioral tests were applied to evaluate the depression model; immunofluorescence and immunohistochemistry staining were used to explore morphological changes in astrocytes; astrocyte-specific RNA sequencing (RNA-Seq) analysis was performed to capture transcriptome wide alterations; and ATP and oxygen consumption rate (OCR) levels of primary astrocytes were measured, followed by YL-0919 incubation to appraise the alteration of energy metabolism and mitochondrial oxidative phosphorylation (OXPHOS). Results: YL-0919 alleviated CRS-induced depressive-like behaviors faster than fluoxetine and attenuated the number and morphologic deficits in the astrocytes of depressed mice. The changes of gene expression profile in astrocytes after CRS were partially reversed by YL-0919. Moreover, YL-0919 improved astrocyte energy metabolism and mitochondrial OXPHOS in astrocytes. Conclusion: Our results provide evidence that YL-0919 exerted a faster-onset antidepressant effect on CRS-mice possibly via astrocyte structural remodeling and mitochondria functional restoration.
Keywords: RNA-seq; YL-0919; astrocytes; depression; mitochondria.
Copyright © 2023 Li, Hu, Chang, Bao, Kong, Ma and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Astrocyte-specific activation of sigma-1 receptors in mPFC mediates the faster onset antidepressant effect by inhibiting NF-κB-induced neuroinflammation.Brain Behav Immun. 2024 Aug;120:256-274. doi: 10.1016/j.bbi.2024.06.008. Epub 2024 Jun 8. Brain Behav Immun. 2024. PMID: 38852761
-
The astrocytic sigma-1 receptor constitutes in the fast antidepressant action of hypidone hydrochloride (YL-0919) in rodents.Front Pharmacol. 2025 Mar 26;16:1564851. doi: 10.3389/fphar.2025.1564851. eCollection 2025. Front Pharmacol. 2025. PMID: 40206084 Free PMC article.
-
The Faster-Onset Antidepressant Effects of Hypidone Hydrochloride (YL-0919) in Monkeys Subjected to Chronic Unpredictable Stress.Front Pharmacol. 2020 Nov 26;11:586879. doi: 10.3389/fphar.2020.586879. eCollection 2020. Front Pharmacol. 2020. PMID: 33324217 Free PMC article.
-
Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans.Glia. 2021 Sep;69(9):2077-2099. doi: 10.1002/glia.23994. Epub 2021 Mar 18. Glia. 2021. PMID: 33734498 Review.
-
An astroglial basis of major depressive disorder? An overview.Glia. 2017 Aug;65(8):1227-1250. doi: 10.1002/glia.23143. Epub 2017 Mar 20. Glia. 2017. PMID: 28317185 Review.
Cited by
-
Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target.Int J Mol Sci. 2024 Jun 8;25(12):6357. doi: 10.3390/ijms25126357. Int J Mol Sci. 2024. PMID: 38928062 Free PMC article. Review.
-
Hypidone hydrochloride (YL-0919) ameliorates functional deficits after traumatic brain injury in mice by activating the sigma-1 receptor for antioxidation.Neural Regen Res. 2025 Aug 1;20(8):2325-2336. doi: 10.4103/NRR.NRR-D-23-01424. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 39359091 Free PMC article.
-
Human pluripotent stem cells as a translational toolkit in psychedelic research in vitro.iScience. 2024 Mar 28;27(5):109631. doi: 10.1016/j.isci.2024.109631. eCollection 2024 May 17. iScience. 2024. PMID: 38628967 Free PMC article. Review.
-
Hypidone Hydrochloride (YL-0919), a Sigma-1 Receptor Agonist, Improves Attention by Increasing BDNF in mPFC.Pharmaceuticals (Basel). 2025 Mar 24;18(4):455. doi: 10.3390/ph18040455. Pharmaceuticals (Basel). 2025. PMID: 40283892 Free PMC article.
References
-
- Banasr M., Chowdhury G. M., Terwilliger R., Newton S. S., Duman R. S., Behar K. L., et al. (2010). Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 15 (5), 501–511. 10.1038/mp.2008.106 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

