Endothelial KCa channels: Novel targets to reduce atherosclerosis-driven vascular dysfunction
- PMID: 37063294
- PMCID: PMC10102451
- DOI: 10.3389/fphar.2023.1151244
Endothelial KCa channels: Novel targets to reduce atherosclerosis-driven vascular dysfunction
Abstract
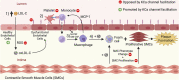
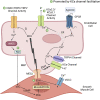
Elevated levels of cholesterol in the blood can induce endothelial dysfunction, a condition characterized by impaired nitric oxide production and decreased vasodilatory capacity. Endothelial dysfunction can promote vascular disease, such as atherosclerosis, where macrophages accumulate in the vascular intima and fatty plaques form that impair normal blood flow in conduit arteries. Current pharmacological strategies to treat atherosclerosis mostly focus on lipid lowering to prevent high levels of plasma cholesterol that induce endothelial dysfunction and atherosclerosis. While this approach is effective for most patients with atherosclerosis, for some, lipid lowering is not enough to reduce their cardiovascular risk factors associated with atherosclerosis (e.g., hypertension, cardiac dysfunction, stroke, etc.). For such patients, additional strategies targeted at reducing endothelial dysfunction may be beneficial. One novel strategy to restore endothelial function and mitigate atherosclerosis risk is to enhance the activity of Ca2+-activated K+ (KCa) channels in the endothelium with positive gating modulator drugs. Here, we review the mechanism of action of these small molecules and discuss their ability to improve endothelial function. We then explore how this strategy could mitigate endothelial dysfunction in the context of atherosclerosis by examining how KCa modulators can improve cardiovascular function in other settings, such as aging and type 2 diabetes. Finally, we consider questions that will need to be addressed to determine whether KCa channel activation could be used as a long-term add-on to lipid lowering to augment atherosclerosis treatment, particularly in patients where lipid-lowering is not adequate to improve their cardiovascular health.
Keywords: KCa channel; SKA-31; atherosclerosis; endothelium; vascular function.
Copyright © 2023 Vera, Wulff and Braun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Administration of the KCa channel activator SKA-31 improves endothelial function in the aorta of atherosclerosis-prone mice.Front Pharmacol. 2025 Feb 28;16:1545050. doi: 10.3389/fphar.2025.1545050. eCollection 2025. Front Pharmacol. 2025. PMID: 40093319 Free PMC article.
-
Pharmacologic targeting of endothelial Ca2+-activated K+ channels: A strategy to improve cardiovascular function.Channels (Austin). 2018 Jan 1;12(1):126-136. doi: 10.1080/19336950.2018.1454814. Channels (Austin). 2018. PMID: 29577810 Free PMC article. Review.
-
Pharmacological Targeting of KCa Channels to Improve Endothelial Function in the Spontaneously Hypertensive Rat.Int J Mol Sci. 2019 Jul 16;20(14):3481. doi: 10.3390/ijms20143481. Int J Mol Sci. 2019. PMID: 31315169 Free PMC article.
-
KCa channel activation normalizes endothelial function in Type 2 Diabetic resistance arteries by improving intracellular Ca2+ mobilization.Metabolism. 2021 Jan;114:154390. doi: 10.1016/j.metabol.2020.154390. Epub 2020 Oct 8. Metabolism. 2021. PMID: 33039407 Free PMC article.
-
Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease.Expert Opin Ther Targets. 2010 Feb;14(2):143-55. doi: 10.1517/14728220903540257. Expert Opin Ther Targets. 2010. PMID: 20055714 Free PMC article. Review.
Cited by
-
Endothelial TRPV4-Cx43 signalling complex regulates vasomotor tone in resistance arteries.J Physiol. 2025 Aug;603(15):4345-4366. doi: 10.1113/JP285194. Epub 2025 Feb 21. J Physiol. 2025. PMID: 39982706 Free PMC article.
-
MicroRNA-19a-3p inhibits endothelial dysfunction in atherosclerosis by targeting JCAD.BMC Cardiovasc Disord. 2024 Jul 30;24(1):394. doi: 10.1186/s12872-024-04063-y. BMC Cardiovasc Disord. 2024. PMID: 39080547 Free PMC article.
-
Novel Insights into the Molecular Mechanisms of Atherosclerosis.Int J Mol Sci. 2023 Aug 30;24(17):13434. doi: 10.3390/ijms241713434. Int J Mol Sci. 2023. PMID: 37686238 Free PMC article. Review.
-
Endothelial Dysfunction in Diabetes Mellitus: New Insights.Int J Mol Sci. 2023 Jun 27;24(13):10705. doi: 10.3390/ijms241310705. Int J Mol Sci. 2023. PMID: 37445881 Free PMC article. Review.
-
N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine induces endothelium-dependent hyperpolarization-mediated vasorelaxation via store-operated calcium entry mechanism in healthy and intestinal inflammatory mice.Heliyon. 2024 Jul 6;10(14):e33994. doi: 10.1016/j.heliyon.2024.e33994. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108891 Free PMC article.
References
-
- Al-Ghananeem A. M., Abbassi M., Shrestha S., Raman G., Wulff H., Pereira L., et al. (2010). Formulation-based approach to support early drug discovery and development efforts: A case study with enteric microencapsulation dosage form development for a triarylmethane derivative TRAM-34; a novel potential immunosuppressant. Drug Dev. Ind. Pharm. 36 (5), 563–569. 10.3109/03639040903329554 - DOI - PMC - PubMed
-
- Ataga K. I., Reid M., Ballas S. K., Yasin Z., Bigelow C., James L. S., et al. (2011). Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: A phase III randomized, placebo-controlled, double-blind study of the gardos channel blocker senicapoc (ICA-17043). Br. J. Haematol. 153 (1), 92–104. 10.1111/j.1365-2141.2010.08520.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

