Role of transporters in regulating mammalian intracellular inorganic phosphate
- PMID: 37063296
- PMCID: PMC10097972
- DOI: 10.3389/fphar.2023.1163442
Role of transporters in regulating mammalian intracellular inorganic phosphate
Abstract
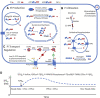
This review summarizes the current understanding of the role of plasma membrane transporters in regulating intracellular inorganic phosphate ([Pi]In) in mammals. Pi influx is mediated by SLC34 and SLC20 Na+-Pi cotransporters. In non-epithelial cells other than erythrocytes, Pi influx via SLC20 transporters PiT1 and/or PiT2 is balanced by efflux through XPR1 (xenotropic and polytropic retrovirus receptor 1). Two new pathways for mammalian Pi transport regulation have been described recently: 1) in the presence of adequate Pi, cells continuously internalize and degrade PiT1. Pi starvation causes recycling of PiT1 from early endosomes to the plasma membrane and thereby increases the capacity for Pi influx; and 2) binding of inositol pyrophosphate InsP8 to the SPX domain of XPR1 increases Pi efflux. InsP8 is degraded by a phosphatase that is strongly inhibited by Pi. Therefore, an increase in [Pi]In decreases InsP8 degradation, increases InsP8 binding to SPX, and increases Pi efflux, completing a feedback loop for [Pi]In homeostasis. Published data on [Pi]In by magnetic resonance spectroscopy indicate that the steady state [Pi]In of skeletal muscle, heart, and brain is normally in the range of 1-5 mM, but it is not yet known whether PiT1 recycling or XPR1 activation by InsP8 contributes to Pi homeostasis in these organs. Data on [Pi]In in cultured cells are variable and suggest that some cells can regulate [Pi] better than others, following a change in [Pi]Ex. More measurements of [Pi]In, influx, and efflux are needed to determine how closely, and how rapidly, mammalian [Pi]In is regulated during either hyper- or hypophosphatemia.
Keywords: SLC20; XPR1; inorganic phosphate; inositol pyrophosphates; regulation; transport.
Copyright © 2023 Jennings.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3568-3574. doi: 10.1073/pnas.1908830117. Epub 2020 Feb 4. Proc Natl Acad Sci U S A. 2020. PMID: 32019887 Free PMC article.
-
Transport and InsP8 gating mechanisms of the human inorganic phosphate exporter XPR1.Nat Commun. 2025 Mar 20;16(1):2770. doi: 10.1038/s41467-025-58076-y. Nat Commun. 2025. PMID: 40113814 Free PMC article.
-
Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis.J Biol Chem. 2020 Jul 10;295(28):9366-9378. doi: 10.1074/jbc.RA119.011376. Epub 2020 May 11. J Biol Chem. 2020. PMID: 32393577 Free PMC article.
-
Phosphate transporters of the SLC20 and SLC34 families.Mol Aspects Med. 2013 Apr-Jun;34(2-3):386-95. doi: 10.1016/j.mam.2012.07.007. Mol Aspects Med. 2013. PMID: 23506879 Review.
-
Sodium-dependent phosphate cotransporters: lessons from gene knockout and mutation studies.J Pharm Sci. 2011 Sep;100(9):3719-30. doi: 10.1002/jps.22614. Epub 2011 May 12. J Pharm Sci. 2011. PMID: 21567407 Review.
Cited by
-
Characterization of the expression of XPR1 in ovine utero-placental tissues.Reproduction. 2025 May 13;169(6):e250072. doi: 10.1530/REP-25-0072. Print 2025 Jun 1. Reproduction. 2025. PMID: 40310868 Free PMC article.
-
The putative polyamine transporter Shp2 facilitates phosphate export in an Xpr1-independent manner and contributes to high phosphate tolerance.J Biol Chem. 2025 Jan;301(1):108056. doi: 10.1016/j.jbc.2024.108056. Epub 2024 Dec 9. J Biol Chem. 2025. PMID: 39662831 Free PMC article.
-
Synergistic activation of the human phosphate exporter XPR1 by KIDINS220 and inositol pyrophosphate.Nat Commun. 2025 Mar 24;16(1):2879. doi: 10.1038/s41467-025-58200-y. Nat Commun. 2025. PMID: 40128258 Free PMC article.
-
Homeostatic coordination of cellular phosphate uptake and efflux requires an organelle-based receptor for the inositol pyrophosphate IP8.Cell Rep. 2024 Jun 25;43(6):114316. doi: 10.1016/j.celrep.2024.114316. Epub 2024 Jun 2. Cell Rep. 2024. PMID: 38833370 Free PMC article.
-
The role of fibroblast growth factor 23 in regulation of phosphate balance.Pediatr Nephrol. 2024 Dec;39(12):3439-3451. doi: 10.1007/s00467-024-06395-5. Epub 2024 Jun 14. Pediatr Nephrol. 2024. PMID: 38874635 Review.
References
-
- Abbasian N., Bevington A., Burton J. O., Herbert K. E., Goodall A. H., Brunskill N. J. (2020a). Inorganic phosphate (Pi) signaling in endothelial cells: A molecular basis for generation of endothelial microvesicles in uraemic cardiovascular disease. Int. J. Mol. Sci. 21, 6993. 10.3390/ijms21196993 - DOI - PMC - PubMed
-
- Abbasian N., Burton J. O., Herbert K. E., Tregunna B. E., Brown J. R., Ghaderi-Najafabadi M., et al. (2015). Hyperphosphatemia, phosphoprotein phosphatases, and microparticle release in vascular endothelial cells. J. Am. Soc. Nephrol. 26, 2152–2162. [doi]. 10.1681/ASN.2014070642 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

