Population pharmacokinetic analysis and dosing optimization of polymyxin B in critically ill patients
- PMID: 37063299
- PMCID: PMC10090446
- DOI: 10.3389/fphar.2023.1122310
Population pharmacokinetic analysis and dosing optimization of polymyxin B in critically ill patients
Abstract
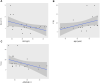
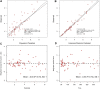
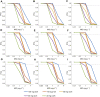
Objectives: Since the global broadcast of multidrug-resistant gram-negative bacteria is accelerating, the use of Polymyxin B is sharply increasing, especially in critically ill patients. Unsatisfactory therapeutic effects were obtained because of the abnormal physiological function in critically ill patients. Therefore, the determination of optimal polymyxin B dosage becomes highly urgent. This study aimed to illustrate the polymyxin B pharmacokinetic characteristics by defining the influencing factors and optimizing the dosing regimens to achieve clinical effectiveness. Methods: Steady-state concentrations of polymyxin B from twenty-two critically ill patients were detected by a verified liquid chromatography-tandem mass spectrometry approach. The information on age, weight, serum creatinine, albumin levels, and Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score was also collected. The population PK parameters were calculated by the non-parametric adaptive grid method in Pmetrics software, and the pharmacokinetic/pharmacodynamics target attainment rate was determined by the Monte Carlo simulation method. Results: The central clearance and apparent volume of distribution for polymyxin B were lower in critically ill patients (1.24 ± 0.38 L h-1 and 16.64 ± 12.74 L, respectively). Moreover, albumin (ALB) levels can be used to explain the variability in clearance, and age can be used to describe the variability in the apparent volume of distribution. For maintaining clinical effectiveness and lowering toxicity, 75 mg q12 h is the recommended dosing regimen for most patients suffering from severe infections. Conclusion: This study has clearly defined that in critically ill patients, age and ALB levels are potentially important factors for the PK parameters of polymyxin B. Since older critically ill patients tend to have lower ALB levels, so higher dosages of polymyxin B are necessary for efficacy.
Keywords: albumin levels; critically ill patients; dosing optimization; polymyxin B; population pharmacokinetics.
Copyright © 2023 Liang, Liang, Deng, Cen, Luo, Zhang and Ni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens.Clin Infect Dis. 2013 Aug;57(4):524-31. doi: 10.1093/cid/cit334. Epub 2013 May 22. Clin Infect Dis. 2013. PMID: 23697744
-
The population pharmacokinetics and dose optimization of polymyxin B in critically ill patients with or without extracorporeal membrane oxygenation.J Clin Pharm Ther. 2022 Oct;47(10):1608-1618. doi: 10.1111/jcpt.13711. Epub 2022 Jun 18. J Clin Pharm Ther. 2022. PMID: 35716048
-
Population Pharmacokinetics of Polymyxin B and Dosage Optimization in Renal Transplant Patients.Front Pharmacol. 2021 Aug 25;12:727170. doi: 10.3389/fphar.2021.727170. eCollection 2021. Front Pharmacol. 2021. PMID: 34512352 Free PMC article.
-
Optimizing use of colistin and polymyxin B in the critically ill.Semin Respir Crit Care Med. 2007 Dec;28(6):604-14. doi: 10.1055/s-2007-996407. Semin Respir Crit Care Med. 2007. PMID: 18095224 Review.
-
Cotrimoxazole - optimal dosing in the critically ill.Ann Intensive Care. 2014 Apr 28;4:13. doi: 10.1186/2110-5820-4-13. eCollection 2014. Ann Intensive Care. 2014. PMID: 24910807 Free PMC article. Review.
Cited by
-
Population pharmacokinetics of polymyxin B in critically ill patients with carbapenem-resistant organisms infections: insights from steady-state trough and peak plasma concentration.Front Pharmacol. 2025 Mar 12;16:1511088. doi: 10.3389/fphar.2025.1511088. eCollection 2025. Front Pharmacol. 2025. PMID: 40144658 Free PMC article.
-
Efficacy, Safety, and Cost-Effectiveness Analysis of Ceftazidime-Avibactam versus Polymyxin B in the Treatment of Carbapenem-Resistant Enterobacteriaceae Infections: A Target Trial Emulation.Infect Dis Ther. 2025 Jul;14(7):1419-1437. doi: 10.1007/s40121-025-01164-9. Epub 2025 May 17. Infect Dis Ther. 2025. PMID: 40381177 Free PMC article.
-
Relationship between plasma polymyxin B concentrations and acute kidney injury in critically ill elderly patients: Findings from a prospective study.J Int Med Res. 2025 Feb;53(2):3000605251320733. doi: 10.1177/03000605251320733. J Int Med Res. 2025. PMID: 39956623 Free PMC article.
-
Comparative pharmacokinetics of polymyxin B in critically ill elderly patients with extensively drug-resistant gram-negative bacteria infections.Front Pharmacol. 2024 Feb 1;15:1347130. doi: 10.3389/fphar.2024.1347130. eCollection 2024. Front Pharmacol. 2024. PMID: 38362145 Free PMC article.
-
How can polymyxin B be dosed based on current pharmacokinetic knowledge?Eur J Clin Pharmacol. 2024 Sep;80(9):1421-1423. doi: 10.1007/s00228-024-03708-3. Epub 2024 Jun 7. Eur J Clin Pharmacol. 2024. PMID: 38847855 No abstract available.
References
-
- Cucci M. D., Gerlach A. T., Mangira C., Murphy C. V., Roberts J. A., Udy A. A., et al. (2022). Performance of different body weights in the cockcroft-gault equation in critically ill patients with and without augmented renal clearance: A multicenter cohort. Pharmacotherapy. 10.1002/phar.2743 - DOI - PMC - PubMed
-
- Falagas M. E., Kyriakidou M., Voulgaris G. L., Vokos F., Politi S., Kechagias K. S. (2021). Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: An evaluation of the current evidence. J. Glob. Antimicrob. Resist 24, 342–359. 10.1016/j.jgar.2020.12.026 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

