High baseline prevalence of atopic comorbidities and medication use in children treated with allergy immunotherapy in the REAl-world effeCtiveness in allergy immunoTherapy (REACT) study
- PMID: 37063677
- PMCID: PMC10098718
- DOI: 10.3389/fped.2023.1136942
High baseline prevalence of atopic comorbidities and medication use in children treated with allergy immunotherapy in the REAl-world effeCtiveness in allergy immunoTherapy (REACT) study
Abstract
Background: Respiratory allergy, commonly manifesting as allergic rhinitis (AR) and asthma, is a chronic progressive disease that frequently starts in childhood. Allergy immunotherapy (AIT) is the only causal treatment for respiratory allergy with the potential to modify the underlying cause of allergy and, ultimately, prevent disease progression. This analysis aimed to determine if AIT is received sufficiently early to halt the progression of allergic disease, by characterizing the burden and progression of disease in children prior to AIT initiation in real-life clinical practice.
Methods: The REAl-world effeCtiveness in allergy immunoTherapy (REACT) study was a large retrospective cohort study using German claims data between 2007 and 2017. Characteristics of two pre-defined AIT age cohorts from the REACT study - children (aged <18 years) and adults (aged ≥18 years) - were evaluated during the 1-year period before the first AIT prescription. For comparison, a control group of all subjects with a confirmed diagnosis of AR and without prescriptions for AIT was included. Burden of disease was assessed using diagnostic codes for atopic comorbidities [e.g., atopic dermatitis (AD), asthma, and acute allergic conjunctivitis] and non-atopic comorbidities (e.g., migraine, headache); medication use, recorded as prescriptions for symptom-relieving AR medication and reliever/controller medication for asthma, was also assessed. Data were analyzed descriptively, using summary statistics.
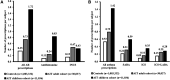
Results: Both children (n = 11,036) and adults (n = 30,037) showed a higher prevalence of atopic comorbidities and a greater drug burden prior to AIT initiation compared to AR patients not treated with AIT (n = 1,003,332). In the two age-specific AIT cohorts, children consistently showed the highest prevalence of atopic comorbidities compared to adults (AIT children, AIT adults - asthma: 41.4%, 34.5%; AD: 19.9%, 10.2%; acute allergic conjunctivitis: 13.6%, 10.2%). Generally, prescriptions per year for symptom-relieving AR and asthma treatments were also higher for children initiating AIT vs. adults (AIT children, AIT adults - AR prescriptions per subject: 1.72, 0.73; asthma prescriptions per subject: 1.42, 0.79).
Conclusions: Children with AR who are offered AIT in real-life show considerable disease burden prior to initiation. As AIT may alleviate the burden and halt the progression of allergic disease, considering AIT earlier in the disease course may be warranted.
Keywords: Allergic rhinitis; allergy immunotherapy; asthma; atopic comorbidities; atopic dermatitis; disease burden; pediatric; real-world evidence (RWE).
© 2023 Fritzsching, Porsbjerg, Buchs, Larsen, Freemantle and Contoli.
Conflict of interest statement
MC reports personal fees from ALK-Abelló; personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Novartis, and Zambon; grants, personal fees and non-financial support from Chiesi and GlaxoSmithKline; and grants from the University of Ferrara, Italy. CP reports grants from ALK-Abelló, and grants and personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Chiesi, Sanofi, and TEVA. SB and JR L are employees of ALK-Abelló. NF reports personal fees from AstraZeneca, Ipsen, Sanofi Aventis, Grifols, Novartis, Aimmune, Vertex, MSD, and Allergan. BF reports personal fees from ALK, and speaker honorarium from Novartis and Merck Sharp & Dohme.
Figures



Similar articles
-
Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: Results from the REACT study, a retrospective cohort study.Lancet Reg Health Eur. 2021 Nov 30;13:100275. doi: 10.1016/j.lanepe.2021.100275. eCollection 2022 Feb. Lancet Reg Health Eur. 2021. PMID: 34901915 Free PMC article.
-
Real-world evidence costs of allergic rhinitis and allergy immunotherapy in the commercially insured United States population.Curr Med Res Opin. 2021 Jun;37(6):957-965. doi: 10.1080/03007995.2021.1903848. Epub 2021 Apr 2. Curr Med Res Opin. 2021. PMID: 33754932
-
Real-world, long-term effectiveness of allergy immunotherapy in allergic rhinitis: Subgroup analyses of the REACT study.J Allergy Clin Immunol. 2023 Aug;152(2):445-452.e4. doi: 10.1016/j.jaci.2023.02.024. Epub 2023 Mar 3. J Allergy Clin Immunol. 2023. PMID: 36871918
-
Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention.Pediatr Allergy Immunol. 2004 Jun;15 Suppl 16:4-5, 9-32. doi: 10.1111/j.1399-3038.2004.0148b.x. Pediatr Allergy Immunol. 2004. PMID: 15125698 Review.
-
Real-world evidence for the long-term effect of allergen immunotherapy: Current status on database-derived European studies.Allergy. 2022 Dec;77(12):3584-3592. doi: 10.1111/all.15506. Epub 2022 Sep 19. Allergy. 2022. PMID: 36074052 Free PMC article. Review.
Cited by
-
Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet (12 SQ-HDM) in children with allergic rhinitis/rhinoconjunctivitis with or without asthma (MT-12): a randomised, double-blind, placebo-controlled, phase III trial.Lancet Reg Health Eur. 2024 Nov 26;48:101136. doi: 10.1016/j.lanepe.2024.101136. eCollection 2025 Jan. Lancet Reg Health Eur. 2024. PMID: 39678704 Free PMC article.
References
-
- Valovirta E. EFA Book on respiratory allergies – raise awareness, relieve the burden. Belgium https://www.efanet.org/images/documents/EFABookonRespiratoryAllergiesFIN... (Accessed 7 July 2022).
-
- Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases One and Three repeat multicountry cross-sectional surveys. Lancet. (2006) 368(9537):733–43. 10.1016/S0140-6736(06)69283-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

