Population pharmacokinetics of vancomycin in very low birth weight neonates
- PMID: 37063687
- PMCID: PMC10101232
- DOI: 10.3389/fped.2023.1093171
Population pharmacokinetics of vancomycin in very low birth weight neonates
Abstract
Introduction: Vancomycin dosing in very low birth weight (VLBW) neonates is challenging. Compared with the general neonatal population, VLBW neonates are less likely to achieve the vancomycin therapeutic targets. Current dosing recommendations are based on studies of the general neonatal population, as only a very limited number of studies have evaluated vancomycin pharmacokinetics in VLBW neonates. The main aim of this study was to develop a vancomycin population pharmacokinetic model to optimize vancomycin dosing in VLBW neonates.
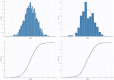
Methods: This multicenter study was conducted at six major hospitals in Saudi Arabia. The study included VLBW neonates who received vancomycin and had at least one vancomycin serum trough concentration measurement at a steady state. We developed a pharmacokinetic model and performed Monte Carlo simulations to develop an optimized dosing regimen for VLBW infants. We evaluated two different targets: AUC0-24 of 400-600 or 400-800 µg. h/mL. We also estimated the probability of trough concentrations >15 and 20 µg/mL.
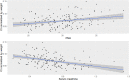
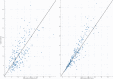
Results: In total, we included 236 neonates, 162 in the training dataset, and 74 in the validation dataset. A one-compartment model was used, and the distribution volume was significantly associated only with weight, whereas clearance was significantly associated with weight, postmenstrual age (PMA), and serum creatinine (Scr).
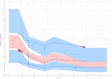
Discussion: We developed dosing regimens for VLBW neonates, considering the probability of achieving vancomycin therapeutic targets, as well as different toxicity thresholds. The dosing regimens were classified according to PMA and Scr. These dosing regimens can be used to optimize the initial dose of vancomycin in VLBW neonates.
Keywords: infectious disease; pharmacodynamic; pharmacokenetics; vancomycin; very low birth weigh neonates.
© 2023 Alsultan, Al Munajem, Atiq, Aljehani, Al Muqati, Almohaizeie, Ballal, Refaei, Al Jeraisy, Assiri and Abouelkheir.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

