Control of the assembly of a cyclic hetero[4]pseudorotaxane from a self-complementary [2]rotaxane
- PMID: 37063802
- PMCID: PMC10094293
- DOI: 10.1039/d3sc00886j
Control of the assembly of a cyclic hetero[4]pseudorotaxane from a self-complementary [2]rotaxane
Abstract
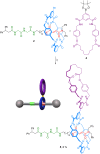
The synthesis of a ditopic interlocked building-block and its self-assembly into a cyclic dimer is reported herein. Starting from a thread with two recognition sites, a three-component clipping reaction was carried out to construct a bistable [2]rotaxane. A subsequent Suzuki cross-coupling reaction allowed the connection of a second ring to that of the rotaxane, affording a self-complementary ditopic system. NMR studies were carried out to identify a cyclic hetero[4]pseudorotaxane as the main supramolecular structure in solution. Its assembly is the result of a positive cooperativity operating in the hydrogen-bonding-driven assembly of this mechanically interlocked supramolecule, as revealed by computational studies. The increase of the polarity of the solvent allows the disruption of the intercomponent interactions and the disassembly of the hetero[4]pseudorotaxane into the two interlocked units. The disassembly of the cyclic dimer was also achieved through a Diels-Alder reaction over the fumaramide binding site of the thread, triggering the translational motion of the entwined macrocycle to an adjacent glycylglycine-based station and precluding the supramolecular dimerization. The competitive molecular recognition of a guest molecule by one of the self-templating counterparts of the dimer also led to the controlled disassembly of the hetero[4]pseudorotaxane.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Kay E. R. Leigh D. A. Angew. Chem., Int. Ed. 2015;54:10080–10088. doi: 10.1002/anie.201503375. - DOI - PMC - PubMed
- Sauvage J.-P. Angew. Chem., Int. Ed. 2017;56:11080–11093. doi: 10.1002/anie.201702992. - DOI - PubMed
- Stoddart J. F. Angew. Chem., Int. Ed. 2017;56:11094–11125. doi: 10.1002/anie.201703216. - DOI - PubMed
-
- Zhang L. Marcos V. Leigh D. A. Proc. Natl. Acad. Sci. U. S. A. 2018;115:9397–9404. doi: 10.1073/pnas.1712788115. - DOI - PMC - PubMed
- Aprahamian I. ACS Cent. Sci. 2020;6:347–358. doi: 10.1021/acscentsci.0c00064. - DOI - PMC - PubMed
- Shi Z.-T. Zhang Q. Tian H. Qu D.-H. Advanced Intelligent Systems. 2020;2:1900169. doi: 10.1002/aisy.201900169. - DOI
- Feng Y. Ovalle M. Seale J. S. W. Lee C. K. Kim D. J. Astumian R. D. Stoddart J. F. J. Am. Chem. Soc. 2021;143:5569–5591. doi: 10.1021/jacs.0c13388. - DOI - PubMed
-
- Sauvage J.-P. and Garpard P., From Non-Covalent Assemblies to Molecular Machines, Wiley, Weinheim, 2011
- Bruns C. J. and Stoddart J. F., The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, New York, 2016
- Taghavi Shahraki B. Maghsoudi S. Fatahi Y. Rabiee N. Bahadorikhalili S. Dinarvand R. Bagherzadeh M. Verpoort F. Coord. Chem. Rev. 2020;423:213484. doi: 10.1016/j.ccr.2020.213484. - DOI
-
- Harada A. Takashima Y. Yamaguchi H. Chem. Soc. Rev. 2009;38:875–882. doi: 10.1039/B705458K. - DOI - PubMed
- Fang L. Olson M. A. Benítez D. Tkatchouk E. Goddard III W. A. Stoddart J. F. Chem. Soc. Rev. 2010;39:17–29. doi: 10.1039/B917901A. - DOI - PubMed
- Mena-Hernando S. Perez E. M. Chem. Soc. Rev. 2019;48:5016–5032. doi: 10.1039/C8CS00888D. - DOI - PubMed
-
- Berna J. Leigh D. A. Lubomska M. Mendoza S. M. Perez E. M. Rudolf P. Teobaldi G. Zerbetto F. Nat. Mater. 2005;4:704–710. doi: 10.1038/nmat1455. - DOI - PubMed
- Green J. E. Choi J. W. Boukai A. Bunimovich Y. Johnston-Halperin E. DeIonno E. Luo Y. Sheriff B. A. Xu K. Shin Y. S. Tseng H.-R. Stoddart J. F. Heath J. R. Nature. 2007;445:414–417. doi: 10.1038/nature05462. - DOI - PubMed
- Lin Q. Hou X. Ke C. Angew. Chem., Int. Ed. 2017;56:4452–4457. doi: 10.1002/anie.201612440. - DOI - PubMed
- Saura-Sanmartin A. Martinez-Cuezva A. Bautista D. Marzari M. R. B. Martins M. A. P. Alajarin M. Berna J. J. Am. Chem. Soc. 2020;142:13442–13449. doi: 10.1021/jacs.0c04477. - DOI - PubMed
LinkOut - more resources
Full Text Sources

